Jim Stertz, Director of Quality/Technology for Lowell Inc.01.19.12
In today’s tough economy, many domestic manufacturers face intense competition for business, not only against other U.S. manufacturers, but also increasingly against offshore sources. How do manufacturing companies maintain an edge in the race to keep and attract business? One option, of course, is automation. Automation isn’t a cure-all, however, and can cause internal conflict if it’s seen as a replacement for skilled employees. It also can be expensive. But if done correctly, automation can be a win-win option. We found this to be true when we turned to automation—not to replace workers, but to enhance their efficiency and output, and make the company more competitive.
We did all our own research and implemented the changes ourselves.
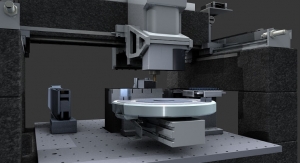
In 2007, Lowell invested in a sophisticated precision measuring machine (PMM) from Leitz of Wetzlar, Germany (this same equipment is used to calibrate gages at the National Institute of Standards and Technology (NIST), which adds inspection capacity to the firm’s existing Brown & Sharpe coordinate measurement machines (CMM). To help the equipment operate at peak accuracy, it’s housed in a 450-square-foot, climate-controlled space that maintains a temperature variation of +/-1° F. However, the equipment was outpacing the inspectors, and they couldn’t keep up with demand, despite doubling capacity. The problem was quickly identified. These two highly sophisticated and very expensive pieces of equipment sat idle while associates loaded and unloaded parts into the machines by hand. The downtime meant that fewer parts were inspected and both customers and machinists waited. Clearly this was not the fault of the operators. After all, humans can only work so fast before they tire, or worse, start to make mistakes. But what about robots?
This question led to an 18-month-long journey to automate the two key pieces of inspection equipment. Now instead of an associate laboriously tending the machines, a Motoman HP20 robot does the work. The entire project was completed in-house, including selecting the robot. programming its operation and interface with the inspection software, even down to the racking, palletizing and barrier systems that allow the robot to work safely. The robot runs virtually 24/7, freeing the operator to perform higher level tasks such as creating test programs and data analysis. In addition, throughput was increased dramatically. Careful research and some out-of-the-box thinking (we knew of no other companies that automated their CMM), and led to a successful automation strategy that did not reduce the number of employees, but to allowed them to perform higher level tasks and increase productivity. Brad Traczyk, CMM/PMM operator, is one of the many associates at Lowell who has benefited from the robot. He noted that “the robot has changed the way I work. Now I’m an operator and a programmer. My efficiency and satisfaction in my job are way up
Previous methodology – how were parts inspected?
Metrology is known as the science of measurement. As is the case with most scientific pursuits, metrology has benefited from technology. Digital micrometers have replaced analog and optical scanners have replaced simple backlight comparators, all in the pursuit of greater certainty. The first coordinate measurement machines were developed in the 1950s to measure increasingly complex military and aerospace components. These were rudimentary two-axis devices that were controlled by hand. Today’s three-axis CMMs are actually robots themselves, performing hundreds of measurements via direct computer control Unfortunately, many of these new methods, including CMMs, added significantly to the amount of time required to inspect and verify features. In addition, partly because of an increased ability to measure them, features on many components have become smaller and more complex. It is not unusual to see tolerances of ±.0001 (100 millionths of an inch). Prior to automation a CMM operator would load a component into a holding fixture and then start the CMM on its testing routine. Depending on the complexity of the part, the CMM’s touch probe might take dozens or hundreds of data points during scanning. Once the testing was complete, the operator would unload the part and then provide the test data directly to the machinist’s or inspector’s computer workstation or via a hard-copy printout. The testing process itself was not ever an issue. CMMs did their job—testing features during setup, in-process and final inspection. With increased demand, however, the issue became how to use the CMMs more productively.
Generation of the idea
In 2008, with increasing productivity and efficiency in mind, we visited Applied Engineering in Yankton, S.D. Applied Engineering is well known for precision machining of aluminum for defense, aerospace, medical and industrial applications. The organization also is an industry leader in the use of robots and automation in machining. The team at Applied Engineering provided a tour of their facility and showed how they developed robotic systems to automate production equipment for nearly continuous operation The next step for us was how to apply what was learned at Applied Engineering.
It was clear that automation could be very successful in production, but the challenge was how to make it work in an area other than manufacturing. We started with a system learned during our Lean manufacturing journey—process mapping. A team broke down each step in the flow of parts, from engineers to the programmers, machinists and inspectors. A process flow was developed and the team identified a glaring weakness in the system. Machinists and inspectors were waiting—for hours, in some cases—for parts to reach the front of the testing queue. The CMM itself was already automated. The valuable time lost came from a manmade problem. Through no fault of their own, human CMM operators could not keep up with demand. That realization led to the next logical step—automation of the part loading.
Execution of a successful automation plan
We had already spent months automating the software routines for PMMs and CMMs. The existing system took information from the part or component and loaded the metrology software called PC-DMIS for the collection and analysis of the CMM/PMM data points. This was an important first step. Now all the team had to do was to insert a robot into the picture. Applied Engineering used Motoman robots and a system of pallets to move parts from place to place. This seemed like a logical way to proceed, so the pallet system was refined for our needs. Each standard 6.5- x 13.5-inch pallet was fitted with a unique holding fixture and a testing routine was designed for each component. The pallets were set up to be identified by the robot through a binary count proximity sensor. The CMM/PMM touch probe then could verify the pallet through a unique identification device on the pallet. Then the PC-DMIS software was loaded and the testing protocol began. The pallets were staged on two racks. The first was the 24-pallet in/out bay. Parts were staged here with three priorities based on how quickly the parts were need from low to high and the highest priority being next. A manual switch on the robot control panel manages these priorities. This step was extremely important because the CMM/PMM are so widely used for in-process testing. Machinists needed immediate access, or using the “Next” switch, to the prioritize the test equipment to verify features before further processing. The second storage rack was used to stage 120 pallets for processing in the low and high priority steps. The robot can pick pallets from high to low priority using a “first in, first out” system.
Increased efficiency and productivity achieved
Today, the CMM/PMM room and the Motoman robot are under the watchful eye of a former part loader, now trained into an upper level programmer position. A big part of the success of the new automated inspection system was the more productive use of employees, not employee reduction. The programmer spends most of his time writing the exacting test protocols in PC-DMIS, designing test fixtures, and interfacing with the machinist and inspectors to keep the system is running smoothly The automated system has changed the way the entire plant operates. The other goal reached with automation was a significant gain in efficiency. Prior to the robot, Lowell was limited to roughly two-shifts of inspection time. Now the robot and CMM/PMM run about 150 hours a week, an increase of nearly 60 percent, with a target of 165 hours a week. This leaves three hours a week for equipment and server maintenance.
Future – more ways to implement automation
Robotic inspection is just the beginning. As the company continues to look at the efficacy of automation, it is considering additional projects, including automating laser-etching department, cleaning and polishing operations and machine tending. What kind of advice would we give to other manufacturers looking to automate? Do your homework up front. The more programming, flow charting and program requirements work you can do as part of project planning, the better. Don’t become wed to a specific style of robot, racking or security system to maintain flexibility of system design and implementation. And lastly, be open to new ideas and learn as you go.
Jim Stertz is the director of Quality/Technology for Lowell Inc., in Minneapolis, Minn. Jim has led the quality efforts at Lowell for 30 years, including the implementation of the company's ISO 9001:2008 and 13485:2003 Quality Management System. He is a senior member of American Society for Quality and serves on the Automation and IT teams at Lowell. Jim also rides a vintage BMW motorcycle that he plans to replace with a Harley Davidson when he turns 100 years old. He can be reached at jim.stertz@lowellinc.com
We did all our own research and implemented the changes ourselves.
In 2007, Lowell invested in a sophisticated precision measuring machine (PMM) from Leitz of Wetzlar, Germany (this same equipment is used to calibrate gages at the National Institute of Standards and Technology (NIST), which adds inspection capacity to the firm’s existing Brown & Sharpe coordinate measurement machines (CMM). To help the equipment operate at peak accuracy, it’s housed in a 450-square-foot, climate-controlled space that maintains a temperature variation of +/-1° F. However, the equipment was outpacing the inspectors, and they couldn’t keep up with demand, despite doubling capacity. The problem was quickly identified. These two highly sophisticated and very expensive pieces of equipment sat idle while associates loaded and unloaded parts into the machines by hand. The downtime meant that fewer parts were inspected and both customers and machinists waited. Clearly this was not the fault of the operators. After all, humans can only work so fast before they tire, or worse, start to make mistakes. But what about robots?
This question led to an 18-month-long journey to automate the two key pieces of inspection equipment. Now instead of an associate laboriously tending the machines, a Motoman HP20 robot does the work. The entire project was completed in-house, including selecting the robot. programming its operation and interface with the inspection software, even down to the racking, palletizing and barrier systems that allow the robot to work safely. The robot runs virtually 24/7, freeing the operator to perform higher level tasks such as creating test programs and data analysis. In addition, throughput was increased dramatically. Careful research and some out-of-the-box thinking (we knew of no other companies that automated their CMM), and led to a successful automation strategy that did not reduce the number of employees, but to allowed them to perform higher level tasks and increase productivity. Brad Traczyk, CMM/PMM operator, is one of the many associates at Lowell who has benefited from the robot. He noted that “the robot has changed the way I work. Now I’m an operator and a programmer. My efficiency and satisfaction in my job are way up
Previous methodology – how were parts inspected?
Metrology is known as the science of measurement. As is the case with most scientific pursuits, metrology has benefited from technology. Digital micrometers have replaced analog and optical scanners have replaced simple backlight comparators, all in the pursuit of greater certainty. The first coordinate measurement machines were developed in the 1950s to measure increasingly complex military and aerospace components. These were rudimentary two-axis devices that were controlled by hand. Today’s three-axis CMMs are actually robots themselves, performing hundreds of measurements via direct computer control Unfortunately, many of these new methods, including CMMs, added significantly to the amount of time required to inspect and verify features. In addition, partly because of an increased ability to measure them, features on many components have become smaller and more complex. It is not unusual to see tolerances of ±.0001 (100 millionths of an inch). Prior to automation a CMM operator would load a component into a holding fixture and then start the CMM on its testing routine. Depending on the complexity of the part, the CMM’s touch probe might take dozens or hundreds of data points during scanning. Once the testing was complete, the operator would unload the part and then provide the test data directly to the machinist’s or inspector’s computer workstation or via a hard-copy printout. The testing process itself was not ever an issue. CMMs did their job—testing features during setup, in-process and final inspection. With increased demand, however, the issue became how to use the CMMs more productively.
Generation of the idea
In 2008, with increasing productivity and efficiency in mind, we visited Applied Engineering in Yankton, S.D. Applied Engineering is well known for precision machining of aluminum for defense, aerospace, medical and industrial applications. The organization also is an industry leader in the use of robots and automation in machining. The team at Applied Engineering provided a tour of their facility and showed how they developed robotic systems to automate production equipment for nearly continuous operation The next step for us was how to apply what was learned at Applied Engineering.
It was clear that automation could be very successful in production, but the challenge was how to make it work in an area other than manufacturing. We started with a system learned during our Lean manufacturing journey—process mapping. A team broke down each step in the flow of parts, from engineers to the programmers, machinists and inspectors. A process flow was developed and the team identified a glaring weakness in the system. Machinists and inspectors were waiting—for hours, in some cases—for parts to reach the front of the testing queue. The CMM itself was already automated. The valuable time lost came from a manmade problem. Through no fault of their own, human CMM operators could not keep up with demand. That realization led to the next logical step—automation of the part loading.
Execution of a successful automation plan
We had already spent months automating the software routines for PMMs and CMMs. The existing system took information from the part or component and loaded the metrology software called PC-DMIS for the collection and analysis of the CMM/PMM data points. This was an important first step. Now all the team had to do was to insert a robot into the picture. Applied Engineering used Motoman robots and a system of pallets to move parts from place to place. This seemed like a logical way to proceed, so the pallet system was refined for our needs. Each standard 6.5- x 13.5-inch pallet was fitted with a unique holding fixture and a testing routine was designed for each component. The pallets were set up to be identified by the robot through a binary count proximity sensor. The CMM/PMM touch probe then could verify the pallet through a unique identification device on the pallet. Then the PC-DMIS software was loaded and the testing protocol began. The pallets were staged on two racks. The first was the 24-pallet in/out bay. Parts were staged here with three priorities based on how quickly the parts were need from low to high and the highest priority being next. A manual switch on the robot control panel manages these priorities. This step was extremely important because the CMM/PMM are so widely used for in-process testing. Machinists needed immediate access, or using the “Next” switch, to the prioritize the test equipment to verify features before further processing. The second storage rack was used to stage 120 pallets for processing in the low and high priority steps. The robot can pick pallets from high to low priority using a “first in, first out” system.
Increased efficiency and productivity achieved
Today, the CMM/PMM room and the Motoman robot are under the watchful eye of a former part loader, now trained into an upper level programmer position. A big part of the success of the new automated inspection system was the more productive use of employees, not employee reduction. The programmer spends most of his time writing the exacting test protocols in PC-DMIS, designing test fixtures, and interfacing with the machinist and inspectors to keep the system is running smoothly The automated system has changed the way the entire plant operates. The other goal reached with automation was a significant gain in efficiency. Prior to the robot, Lowell was limited to roughly two-shifts of inspection time. Now the robot and CMM/PMM run about 150 hours a week, an increase of nearly 60 percent, with a target of 165 hours a week. This leaves three hours a week for equipment and server maintenance.
Future – more ways to implement automation
Robotic inspection is just the beginning. As the company continues to look at the efficacy of automation, it is considering additional projects, including automating laser-etching department, cleaning and polishing operations and machine tending. What kind of advice would we give to other manufacturers looking to automate? Do your homework up front. The more programming, flow charting and program requirements work you can do as part of project planning, the better. Don’t become wed to a specific style of robot, racking or security system to maintain flexibility of system design and implementation. And lastly, be open to new ideas and learn as you go.
Jim Stertz is the director of Quality/Technology for Lowell Inc., in Minneapolis, Minn. Jim has led the quality efforts at Lowell for 30 years, including the implementation of the company's ISO 9001:2008 and 13485:2003 Quality Management System. He is a senior member of American Society for Quality and serves on the Automation and IT teams at Lowell. Jim also rides a vintage BMW motorcycle that he plans to replace with a Harley Davidson when he turns 100 years old. He can be reached at jim.stertz@lowellinc.com