Sam Brusco, Associate Editor10.04.23
Microsure, a Netherlands-based robot-assisted microsurgery company, closed a Series B2 investment of €38 million in development capital. The financing round featured a host of new investors, including the European Innovation Council Fund (EICF), kineo, Invest-NL, and several prominent private investor groups.
Microsure said the funding will be used to finalize the MUSA-3 microsurgical robot’s development for clinical studies, followed by U.S. Food and Drug Administration (FDA) and CE mark clearance.
The company says features like enhanced dexterity, a wide workspace, tremor reduction, and integration with surgeons’ preferred micro-instruments were designed to augment precision, stability, and control during these surgeries. Surgeons can also work from a console with digital exoscopes or hybrid surgical microscopes.
The company also said MUSA-3’s adaptability will allow its use in a wide range of surgical procedures.
"We extend our deep gratitude to both our new and steadfast existing investors for their unwavering support, which has propelled Microsure to new heights,” the company’s CEO Sjaak Deckers told the press. “The financing will enable us to advance our groundbreaking technology and bring innovative solutions to patients worldwide through our MUSA-3 robotic system."
Microsure’s clinical MUSA-2 prototype underwent two recent clinical studies, yielding promising results and valuable insights for MUSA-3’s development. 50 patients underwent a variety of surgical indications at the Netherlands’ Maastricht University Medical Center and Sweden’s Uppsala Academic Hospital. Further, an Uppsala in-vitro study featured 20 microsurgeons performing more than 200 anastomoses, revealing a swift learning curve.

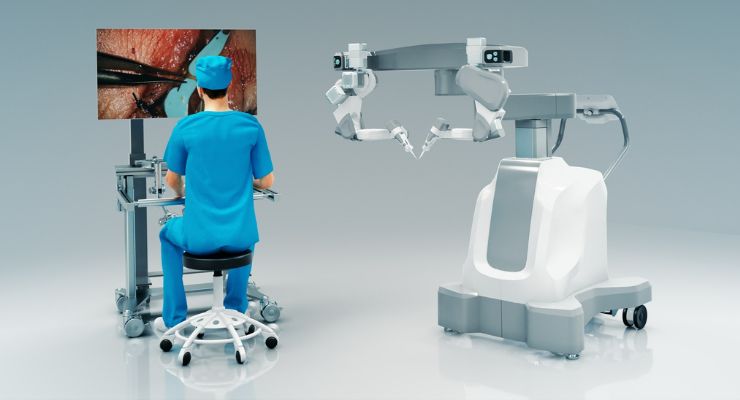
MUSA-2 combined with the Olympus ORBEYE 4K 3D surgical video microscope, during an extremity free flap procedure performed at Maastricht University Medical Center MUMC+.
The company’s first-generation MUSA microsurgery robot secured EU approval in June 2019.
Microsure said the funding will be used to finalize the MUSA-3 microsurgical robot’s development for clinical studies, followed by U.S. Food and Drug Administration (FDA) and CE mark clearance.
The company says features like enhanced dexterity, a wide workspace, tremor reduction, and integration with surgeons’ preferred micro-instruments were designed to augment precision, stability, and control during these surgeries. Surgeons can also work from a console with digital exoscopes or hybrid surgical microscopes.
The company also said MUSA-3’s adaptability will allow its use in a wide range of surgical procedures.
"We extend our deep gratitude to both our new and steadfast existing investors for their unwavering support, which has propelled Microsure to new heights,” the company’s CEO Sjaak Deckers told the press. “The financing will enable us to advance our groundbreaking technology and bring innovative solutions to patients worldwide through our MUSA-3 robotic system."
Microsure’s clinical MUSA-2 prototype underwent two recent clinical studies, yielding promising results and valuable insights for MUSA-3’s development. 50 patients underwent a variety of surgical indications at the Netherlands’ Maastricht University Medical Center and Sweden’s Uppsala Academic Hospital. Further, an Uppsala in-vitro study featured 20 microsurgeons performing more than 200 anastomoses, revealing a swift learning curve.

MUSA-2 combined with the Olympus ORBEYE 4K 3D surgical video microscope, during an extremity free flap procedure performed at Maastricht University Medical Center MUMC+.
The company’s first-generation MUSA microsurgery robot secured EU approval in June 2019.