Michael Barbella, Managing Editor09.22.23
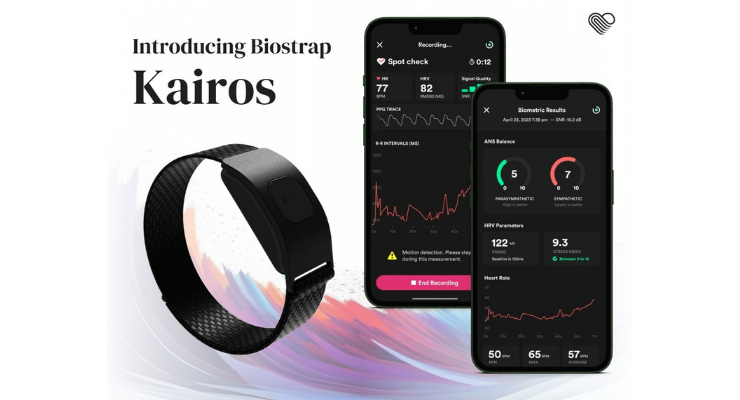
Biostrap USA LLC has launched its new wearable device, Biostrap Kairos. Accompanied by the new Vital Science app, Biostrap Kairos introduces a data visualization of the autonomic nervous system, offering quantification of the sympathetic and parasympathetic branches from a wrist-worn device. Such measurements previously were only available to researchers while using uncomfortable electrocardiogram chest straps.
Biostrap has achieved a significant milestone by delivering a comprehensive stress resilience measurement that allows users to understand the extent to which they are in fight-or-flight or rest-and-digest mode during periods of rest, throughout the day and in real time.
"We are thrilled to launch Kairos and our new Spot Check feature," Biostrap CEO/Co-Founder Sameer Sontakey said. "Our team has worked to create this culmination of hardware and software that positions Biostrap as the leader in quantifying clinical-grade heart rate variability (HRV) parameters and providing researchers and healthtech visionaries insights into their patients' and clients' autonomic nervous system like never before."
Biostrap's team developed Kairos from the ground up, incorporating years of customer feedback. It is a wrist-worn device but has a modular design allowing it to also be positioned on the forearm or bicep.
Utilizing a high-sensitivity complementary metal-oxide-semiconductor (CMOS) optical sensor to capture raw photoplethysmography (PPG) data, Kairos computes biometrics such as active and resting heart rate, HRV, beat-to-beat intervals, respiratory rate as well as sleep-related parameters with accuracy.
"Our highly sensitive CMOS pixel provides high-quality raw PPG signal with excellent signal-to-noise ratio, and that is where PixArt can help Biostrap stand out in the accuracy of biometrics for the enterprise and B2B market," stated Charles Chong, director of strategic marketing at PixArt Imaging USA Inc.
Additionally, utilizing technology from Ambiq, Kairos offers a four times improvement in battery life and 10 times improvement in syncing speeds compared to previous Biostrap devices.
"Ambiq's leadership in low-power microcontrollers utilizing SPOT technology allows Biostrap's first-of-its-kind configurable biometric monitoring platform to have extended battery life, while continuously monitoring health sensors. The combination of our technologies will allow medical professionals to extend professional care beyond facility walls," noted Mike Kenyon, vice president of sales and business development at Ambiq.
Biostrap's key differentiator in the industry is its commitment to delivering clinically reliable nervous system analysis through detailed and transparent biometric measurements, such as the new Spot Check feature. By offering a real-time visualization of the underlying PPG signal on which these biometrics are derived, signal quality, and R-R intervals, Biostrap provides untethered access to every data point captured via the Vital Science app, a Remote Monitoring dashboard, APIs or SDKs.
It is the platform's configurability and data integrity that attracted the National Police Wellbeing Service (NPWS) in the United Kingdom to choose Biostrap in their efforts to improve police officers' health and safety.
"The differentiator with Biostrap is the company's willingness to adapt how it presents data and information to users based on their experiences and our priority issue which is sleep, fatigue and recovery," said Andy Rhodes, director at NPWS and former chief constable with more than 30 years in policing. "We wanted an end-to-end solution which is tuned into the reality of emergency responder work, and Biostrap has listened to frontline officers to achieve that. The accuracy of Biostrap means we can rely on the key data points and present them to officers with a high degree of confidence. It's why we are confident that our sleep, fatigue and recovery programme has been developed by policing for policing."
Kairos underwent extensive testing by key thought leaders in heart rate variability research before its official launch. Data scientist and world-renowned HRV expert Marco Altini, Ph.D., compared Kairos against an ECG chest strap heart rate monitor to assess accuracy levels. In his blog, he said "I was very happy with the data quality of this sensor [Kairos]. This is yet another demonstration that PPG can be used to estimate HRV and that pulse rate variability (i.e. HRV from PPG), when measured at rest in healthy individuals, provides all we need."
The launch of Kairos and the Vital Science app marks Biostrap's official pivot to exit the consumer wearable market and step into offering configurable hardware and software made for enterprise and research purposes.
Biostrap provides scalable and customizable biosensor-based precision health monitoring solutions for visionaries and researchers. The Biostrap suite of devices capture clinical-grade, continuous, high-fidelity raw data for analysis of overall health and well-being. Built with convenience, efficiency, and security in mind, Biostrap translates complex biometric signals into customized insights, dashboards, and mobile applications to meet clients' needs. The Biostrap experience is fully configurable and API and Bluetooth SDK integration options are available upon request.
Biostrap has achieved a significant milestone by delivering a comprehensive stress resilience measurement that allows users to understand the extent to which they are in fight-or-flight or rest-and-digest mode during periods of rest, throughout the day and in real time.
"We are thrilled to launch Kairos and our new Spot Check feature," Biostrap CEO/Co-Founder Sameer Sontakey said. "Our team has worked to create this culmination of hardware and software that positions Biostrap as the leader in quantifying clinical-grade heart rate variability (HRV) parameters and providing researchers and healthtech visionaries insights into their patients' and clients' autonomic nervous system like never before."
Biostrap's team developed Kairos from the ground up, incorporating years of customer feedback. It is a wrist-worn device but has a modular design allowing it to also be positioned on the forearm or bicep.
Utilizing a high-sensitivity complementary metal-oxide-semiconductor (CMOS) optical sensor to capture raw photoplethysmography (PPG) data, Kairos computes biometrics such as active and resting heart rate, HRV, beat-to-beat intervals, respiratory rate as well as sleep-related parameters with accuracy.
"Our highly sensitive CMOS pixel provides high-quality raw PPG signal with excellent signal-to-noise ratio, and that is where PixArt can help Biostrap stand out in the accuracy of biometrics for the enterprise and B2B market," stated Charles Chong, director of strategic marketing at PixArt Imaging USA Inc.
Additionally, utilizing technology from Ambiq, Kairos offers a four times improvement in battery life and 10 times improvement in syncing speeds compared to previous Biostrap devices.
"Ambiq's leadership in low-power microcontrollers utilizing SPOT technology allows Biostrap's first-of-its-kind configurable biometric monitoring platform to have extended battery life, while continuously monitoring health sensors. The combination of our technologies will allow medical professionals to extend professional care beyond facility walls," noted Mike Kenyon, vice president of sales and business development at Ambiq.
Biostrap's key differentiator in the industry is its commitment to delivering clinically reliable nervous system analysis through detailed and transparent biometric measurements, such as the new Spot Check feature. By offering a real-time visualization of the underlying PPG signal on which these biometrics are derived, signal quality, and R-R intervals, Biostrap provides untethered access to every data point captured via the Vital Science app, a Remote Monitoring dashboard, APIs or SDKs.
It is the platform's configurability and data integrity that attracted the National Police Wellbeing Service (NPWS) in the United Kingdom to choose Biostrap in their efforts to improve police officers' health and safety.
"The differentiator with Biostrap is the company's willingness to adapt how it presents data and information to users based on their experiences and our priority issue which is sleep, fatigue and recovery," said Andy Rhodes, director at NPWS and former chief constable with more than 30 years in policing. "We wanted an end-to-end solution which is tuned into the reality of emergency responder work, and Biostrap has listened to frontline officers to achieve that. The accuracy of Biostrap means we can rely on the key data points and present them to officers with a high degree of confidence. It's why we are confident that our sleep, fatigue and recovery programme has been developed by policing for policing."
Kairos underwent extensive testing by key thought leaders in heart rate variability research before its official launch. Data scientist and world-renowned HRV expert Marco Altini, Ph.D., compared Kairos against an ECG chest strap heart rate monitor to assess accuracy levels. In his blog, he said "I was very happy with the data quality of this sensor [Kairos]. This is yet another demonstration that PPG can be used to estimate HRV and that pulse rate variability (i.e. HRV from PPG), when measured at rest in healthy individuals, provides all we need."
The launch of Kairos and the Vital Science app marks Biostrap's official pivot to exit the consumer wearable market and step into offering configurable hardware and software made for enterprise and research purposes.
Biostrap provides scalable and customizable biosensor-based precision health monitoring solutions for visionaries and researchers. The Biostrap suite of devices capture clinical-grade, continuous, high-fidelity raw data for analysis of overall health and well-being. Built with convenience, efficiency, and security in mind, Biostrap translates complex biometric signals into customized insights, dashboards, and mobile applications to meet clients' needs. The Biostrap experience is fully configurable and API and Bluetooth SDK integration options are available upon request.