Sam Brusco, Associate Editor06.08.23
Endologix has gained U.S. Food and Drug Administration (FDA) approval for its DETOUR system to treat patients with complex peripheral arterial disease (PAD).
The recommended therapy for PAD is open surgical bypass, which is invasive, has a high early complication rate, and needs prolonged recovery. Endovascular treatments are feasible in some patients, but have limited patency.
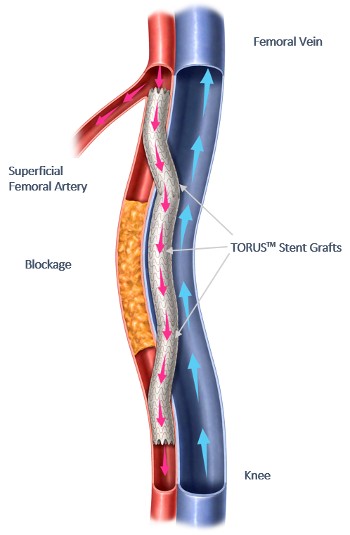
Percutaneous transmural arterial bypass (PTAB) using the DETOUR System. Image courtesy of Business Wire.
Percutaneous transmural arterial bypass (PTAB) using the DETOUR system is a novel approach to treat complex PAD, enabling doctors to bypass lesions in the superficial femoral artery with stents that are routed through the femoral vein to restore blood flow in the leg.
The approach is quite effective for patients with lesions 20-46 cm in length, those that have already had failed endovascular procedures, or those that might be sub-optimal candidates for open surgical bypass.
"We are delighted to receive FDA approval of the DETOUR System," Matt Thompson, MD, president, and CEO of Endologix told the press. “PTAB therapy represents a significant step forward for patients with complex PAD, they have long needed a more effective and less invasive treatment option for long lesions of the SFA. We are proud to be pioneering this novel approach and continuing to innovate on behalf of patients. We look forward to launching this new therapy in the U.S. through a targeted market release in the coming weeks."
The recommended therapy for PAD is open surgical bypass, which is invasive, has a high early complication rate, and needs prolonged recovery. Endovascular treatments are feasible in some patients, but have limited patency.

Percutaneous transmural arterial bypass (PTAB) using the DETOUR System. Image courtesy of Business Wire.
The approach is quite effective for patients with lesions 20-46 cm in length, those that have already had failed endovascular procedures, or those that might be sub-optimal candidates for open surgical bypass.
"We are delighted to receive FDA approval of the DETOUR System," Matt Thompson, MD, president, and CEO of Endologix told the press. “PTAB therapy represents a significant step forward for patients with complex PAD, they have long needed a more effective and less invasive treatment option for long lesions of the SFA. We are proud to be pioneering this novel approach and continuing to innovate on behalf of patients. We look forward to launching this new therapy in the U.S. through a targeted market release in the coming weeks."












