Charles Sternberg, Assistant Editor03.23.21
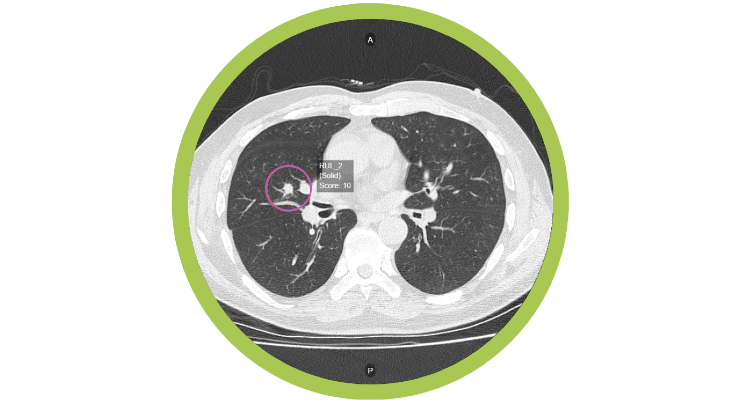
Optellum, a lung health company aiming to redefine early diagnosis and treatment of lung disease, has received FDA 510(k) clearance for its Virtual Nodule Clinic, an AI-powered clinical decision support software for pulmonologists and radiologists managing patients with small lesions in the lungs called nodules that could represent early-stage lung cancer. This is the first such application of AI decision support for early lung cancer diagnosis cleared by the FDA.
Lung cancer kills more people than any other cancer. The current five-year survival rate is an abysmal 20%, primarily due to the majority of patients being diagnosed after symptoms have appeared and the disease has progressed to an advanced stage (Stage III or IV).1 By comparison, the survival rate for small tumors treated at Stage IA is up to 90%.2 The U.S. Preventive Services Task Force (USPSTF) updated theirscreening recommendations earlier this month with a push to diagnose lung cancer earlier when it is more amenable to treatment.
One of greatest opportunities to diagnose more small pre-symptomatic lung cancers earlier is presented by the two million patients in the United States every year3 who have a lung nodule identified incidentally during chest CT scans ordered for other reasons, such as emergency room or cardiac scans. Current guidelines mandate follow-up over one to two years to determine whether a nodule is cancerous. However, over 60% of these patients do not receive guideline-recommended follow-up,4 severely limiting opportunities for early intervention and treatment. Patients who do receive recommended follow-up often require multiple imaging scans and biopsies, and sometimes unnecessary invasive procedures including surgical biopsies and lung resections, before arriving at a definite diagnosis.
Identifying Lung Cancer Risk
Optellum’s Virtual Nodule Clinic is designed to solve this problem by enabling pulmonologists to identify and track at-risk patients with suspicious lung nodules and make optimal clinical management decisions for those patients. The software features a clinically-validated Lung Cancer Prediction (LCP) score designed to empower clinicians to more accurately and consistently evaluate lung cancer risk and make more optimal clinical decisions that could save more patient lives.
Optellum’s LCP score is powered by the world’s first FDA-cleared imaging AI/”Radiomics”-based digital biomarker for lung cancer. The score is computed from full patterns of 3D pixels in standard images captured by Computed Tomography (CT) scanners, which are already available and the standard of care in every modern hospital.
Study Results
Physician use of Virtual Nodule Clinic is shown to improve diagnostic accuracy and clinical decision-making. In the clinical study which underpins the FDA clearance, all readers in the study, which included pulmonologists and radiologists of various levels of expertise, from generalists to experts, showed a statistically significant improvement in their accuracy for diagnosing lung nodules when using the Optellum software, with an average improvement of 6.85 Area Under the Curve (AUC) points (p<0.001) and a range of 2.4 to 12.1 AUC points for an individual physician. In this study, AUC measured readers’ average accuracy in correctly classifying nodules as malignant or benign. In addition to improved diagnostic accuracy, use of the Optellum software resulted in more consistency among physicians.5
“This study demonstrates the clinical impact of the LCP score,” said Dr. Anil Vachani, principal investigator of the study and associate professor and co-director, Lung Cancer Screening at the University of Pennsylvania. “When using the LCP score, all readers in the study significantly improved both their sensitivity and specificity of diagnosis, and readers at all levels of expertise became more consistent. This is significant because it could assist with early lung cancer diagnosis and intervention in today’s clinical practice, where many patients with cancerous nodules may face delays in diagnosis and treatment, while patients with benign nodules are often unnecessarily exposed to aggressive procedures with sometimes life-threatening complications.”
Optellum’s LCP has been extensively validated in additional multi-center studies led by co-authors of clinical guidelines,6 and shown to consistently outperform conventional risk prediction models recommended in the current clinical guidelines and considered state-of-the-art in classifying nodules as low (likely benign), intermediate or high risk (likely cancerous). In an independent validation study led by physicians from Vanderbilt and Oxford, the AI was shown to correctly reclassify indeterminate nodules into high- and low-risk categories in more than a third of cancers and benign nodules,7 illustrating the potential to speed up lung cancer diagnosis and reduce invasive biopsies and surgeries on patients without lung cancer, compared to the current standard of care.
“We are delighted to launch the world’s first AI-based decision support for early lung cancer diagnosis cleared by the FDA,” said Václav Potěšil, Ph.D., co-founder and CEO of Optellum. “This clearance will ensure clinicians have the clinical decision support they need to diagnose and treat lung cancer at the earliest possible stage, harnessing the power of physicians and AI working together – to the benefit of patients. Our goal at Optellum is to redefine early diagnosis and treatment of lung cancer, and this FDA clearance is the first step on that journey. We look forward to empowering clinicians in every hospital, from our current customers at academic medical centers to local community hospitals, to offer patients with lung cancer and other deadly lung diseases the most optimal diagnosis and treatment.”
References:
1 Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: SEER 18 2010–2016, All Races, Both Sexes. Available at https://seer.cancer.gov/statfacts/html/lungb.html
2 AJCC Lung Cancer Staging manual (7th edition)
3 Optellum projections based on Gould MK, Tang T, Liu IL, Lee J, Zheng C, Danforth KN, Kosco AE, Di Fiore JL, Suh DE. Recent Trends in the Identification of Incidental Pulmonary Nodules. Am J Respir Crit Care Med. 2015 Nov 15;192(10):1208-14
4 Pyenson BS, Bazell CM, Bellanich MJ, Caplen MA, Zulueta JJ. No Apparent Workup for most new Indeterminate Pulmonary Nodules in US Commercially-Insured Patients. JHEOR. 2019;6(3):118-129.
5 Vachani A, Massion PP, Munden RF, et al., Imaging AI/”Radiomics” decision support improves physicians’ stratification of indeterminate pulmonary nodules: An MRMC study presented to the American Cancer Society National Lung Cancer Roundtable (NLCRT) 2020. https://vimeo.com/487367357
6 Baldwin DR, Gustafson J, Pickup L, et al. External validation of a convolutional neural network artificial intelligence tool to predict malignancy in pulmonary nodules. Thorax. 2020 Apr;75(4):306-312. https://thorax.bmj.com/content/thoraxjnl/75/4/306.full.pdf
7 Massion PP, Antic S, Ather S, et al. Assessing the Accuracy of a Deep Learning Method to Risk Stratify Indeterminate Pulmonary Nodules. Am J Respir Crit Care Med. 2020 Jul 15;202(2):241-249. https://www.atsjournals.org/doi/full/10.1164/rccm.201903-0505OC
Lung cancer kills more people than any other cancer. The current five-year survival rate is an abysmal 20%, primarily due to the majority of patients being diagnosed after symptoms have appeared and the disease has progressed to an advanced stage (Stage III or IV).1 By comparison, the survival rate for small tumors treated at Stage IA is up to 90%.2 The U.S. Preventive Services Task Force (USPSTF) updated theirscreening recommendations earlier this month with a push to diagnose lung cancer earlier when it is more amenable to treatment.
One of greatest opportunities to diagnose more small pre-symptomatic lung cancers earlier is presented by the two million patients in the United States every year3 who have a lung nodule identified incidentally during chest CT scans ordered for other reasons, such as emergency room or cardiac scans. Current guidelines mandate follow-up over one to two years to determine whether a nodule is cancerous. However, over 60% of these patients do not receive guideline-recommended follow-up,4 severely limiting opportunities for early intervention and treatment. Patients who do receive recommended follow-up often require multiple imaging scans and biopsies, and sometimes unnecessary invasive procedures including surgical biopsies and lung resections, before arriving at a definite diagnosis.
Identifying Lung Cancer Risk
Optellum’s Virtual Nodule Clinic is designed to solve this problem by enabling pulmonologists to identify and track at-risk patients with suspicious lung nodules and make optimal clinical management decisions for those patients. The software features a clinically-validated Lung Cancer Prediction (LCP) score designed to empower clinicians to more accurately and consistently evaluate lung cancer risk and make more optimal clinical decisions that could save more patient lives.
Optellum’s LCP score is powered by the world’s first FDA-cleared imaging AI/”Radiomics”-based digital biomarker for lung cancer. The score is computed from full patterns of 3D pixels in standard images captured by Computed Tomography (CT) scanners, which are already available and the standard of care in every modern hospital.
Study Results
Physician use of Virtual Nodule Clinic is shown to improve diagnostic accuracy and clinical decision-making. In the clinical study which underpins the FDA clearance, all readers in the study, which included pulmonologists and radiologists of various levels of expertise, from generalists to experts, showed a statistically significant improvement in their accuracy for diagnosing lung nodules when using the Optellum software, with an average improvement of 6.85 Area Under the Curve (AUC) points (p<0.001) and a range of 2.4 to 12.1 AUC points for an individual physician. In this study, AUC measured readers’ average accuracy in correctly classifying nodules as malignant or benign. In addition to improved diagnostic accuracy, use of the Optellum software resulted in more consistency among physicians.5
“This study demonstrates the clinical impact of the LCP score,” said Dr. Anil Vachani, principal investigator of the study and associate professor and co-director, Lung Cancer Screening at the University of Pennsylvania. “When using the LCP score, all readers in the study significantly improved both their sensitivity and specificity of diagnosis, and readers at all levels of expertise became more consistent. This is significant because it could assist with early lung cancer diagnosis and intervention in today’s clinical practice, where many patients with cancerous nodules may face delays in diagnosis and treatment, while patients with benign nodules are often unnecessarily exposed to aggressive procedures with sometimes life-threatening complications.”
Optellum’s LCP has been extensively validated in additional multi-center studies led by co-authors of clinical guidelines,6 and shown to consistently outperform conventional risk prediction models recommended in the current clinical guidelines and considered state-of-the-art in classifying nodules as low (likely benign), intermediate or high risk (likely cancerous). In an independent validation study led by physicians from Vanderbilt and Oxford, the AI was shown to correctly reclassify indeterminate nodules into high- and low-risk categories in more than a third of cancers and benign nodules,7 illustrating the potential to speed up lung cancer diagnosis and reduce invasive biopsies and surgeries on patients without lung cancer, compared to the current standard of care.
“We are delighted to launch the world’s first AI-based decision support for early lung cancer diagnosis cleared by the FDA,” said Václav Potěšil, Ph.D., co-founder and CEO of Optellum. “This clearance will ensure clinicians have the clinical decision support they need to diagnose and treat lung cancer at the earliest possible stage, harnessing the power of physicians and AI working together – to the benefit of patients. Our goal at Optellum is to redefine early diagnosis and treatment of lung cancer, and this FDA clearance is the first step on that journey. We look forward to empowering clinicians in every hospital, from our current customers at academic medical centers to local community hospitals, to offer patients with lung cancer and other deadly lung diseases the most optimal diagnosis and treatment.”
References:
1 Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: SEER 18 2010–2016, All Races, Both Sexes. Available at https://seer.cancer.gov/statfacts/html/lungb.html
2 AJCC Lung Cancer Staging manual (7th edition)
3 Optellum projections based on Gould MK, Tang T, Liu IL, Lee J, Zheng C, Danforth KN, Kosco AE, Di Fiore JL, Suh DE. Recent Trends in the Identification of Incidental Pulmonary Nodules. Am J Respir Crit Care Med. 2015 Nov 15;192(10):1208-14
4 Pyenson BS, Bazell CM, Bellanich MJ, Caplen MA, Zulueta JJ. No Apparent Workup for most new Indeterminate Pulmonary Nodules in US Commercially-Insured Patients. JHEOR. 2019;6(3):118-129.
5 Vachani A, Massion PP, Munden RF, et al., Imaging AI/”Radiomics” decision support improves physicians’ stratification of indeterminate pulmonary nodules: An MRMC study presented to the American Cancer Society National Lung Cancer Roundtable (NLCRT) 2020. https://vimeo.com/487367357
6 Baldwin DR, Gustafson J, Pickup L, et al. External validation of a convolutional neural network artificial intelligence tool to predict malignancy in pulmonary nodules. Thorax. 2020 Apr;75(4):306-312. https://thorax.bmj.com/content/thoraxjnl/75/4/306.full.pdf
7 Massion PP, Antic S, Ather S, et al. Assessing the Accuracy of a Deep Learning Method to Risk Stratify Indeterminate Pulmonary Nodules. Am J Respir Crit Care Med. 2020 Jul 15;202(2):241-249. https://www.atsjournals.org/doi/full/10.1164/rccm.201903-0505OC