Penn State11.12.20
An implantable smart wrap that fits safely and securely around the bladder may one day help people who have under-active bladders, a condition that hinders patients from urinating regularly and comfortably, according to an international team of researchers.
In a study, the implant, which combines sensors and a polymer wrap, is an integrated device that can detect when the bladder needs to be completely emptied and then send a signal to a polymer web with an electronic thread that expands or contracts with the bladder. After the bladder is emptied, the band returns to its initial formation.
"Researchers have been interested in studying urinary control for a while because a lot of diseases and conditions are related to this," said Larry Cheng, the Dorothy Quiggle Professor in Engineering and an affiliate of the Institute for Computational and Data Sciences. "There are two conditions in particular that researchers have been studying. The first condition is to force the urine out of the bladder when the muscle might be in a diseased state so that it really can't provide enough force to get the urine out. The second is an overactive bladder, in which an individual experiences the sudden or frequent tendency to urinate, which is related to urinary incontinence."
The device, which was tested in mice, uses sensors to enable precise monitoring in real-time of the bladder to address the under-active bladder condition, he added.
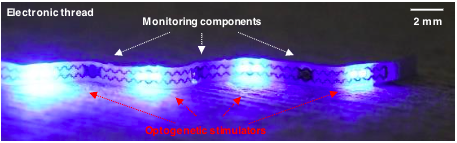
The device can detect when a bladder needs to be completely emptied and then send a signal to a polymer web with an electronic thread that can expand or contract. Image courtesy of Penn State.
Cheng said that past research focused on a mechanical aid to stress the bladder and encourage urination, but the option is difficult to implement and the wrap that surrounded the bladder can slip off. The researchers, who published their findings in Science Advances, said they designed a serpentine-shaped polymer wrap to create a wrap that can stay in place, while expanding to suit the changing shape of the bladder during urination. It can also hold all the necessary sensors and wiring.
"With the serpentine design built into the structure, we can stretch it to a much bigger geometry," said Cheng. "So, if we stretch that serpentine wrap, which is placed around and against the bladder, it would provide a sufficient force to hold the electronic thread with the sensors in place so that it won't be able to slip off."
The microLEDs, which are an array of micro light-emitting diodes, on the electronic thread are designed to deliver light to the bladder for optogenetic neuromodulation, which modulates the function of the targeted organ.
Cheng said that the device is made from materials that are biologically safe and designed to operate for long-time operation in the body. The wrap does not need sutures or glue to be held in place, which is another benefit, he added.
The team used computational resources from ICDS to investigate various designs of the polymer wrap.
"The computational power is really useful because we needed to design this polymer wrap to different geometries," said Cheng. "In this case, we had two different geometries that we investigated. One is a straight line and the other is the serpentine design. And, of course, we tried a few others, so the computational power allowed us to look at different polymer designs and then single out the best one to use."
In the future, the researchers said the wrap might be re-tooled to help people who have unconscious urination due to overactive bladders. The "smart wrap" implant may also help people with other disease conditions to go from diagnostic confirmation to advanced therapeutic options in clinical medicine.
In a study, the implant, which combines sensors and a polymer wrap, is an integrated device that can detect when the bladder needs to be completely emptied and then send a signal to a polymer web with an electronic thread that expands or contracts with the bladder. After the bladder is emptied, the band returns to its initial formation.
"Researchers have been interested in studying urinary control for a while because a lot of diseases and conditions are related to this," said Larry Cheng, the Dorothy Quiggle Professor in Engineering and an affiliate of the Institute for Computational and Data Sciences. "There are two conditions in particular that researchers have been studying. The first condition is to force the urine out of the bladder when the muscle might be in a diseased state so that it really can't provide enough force to get the urine out. The second is an overactive bladder, in which an individual experiences the sudden or frequent tendency to urinate, which is related to urinary incontinence."
The device, which was tested in mice, uses sensors to enable precise monitoring in real-time of the bladder to address the under-active bladder condition, he added.

The device can detect when a bladder needs to be completely emptied and then send a signal to a polymer web with an electronic thread that can expand or contract. Image courtesy of Penn State.
Cheng said that past research focused on a mechanical aid to stress the bladder and encourage urination, but the option is difficult to implement and the wrap that surrounded the bladder can slip off. The researchers, who published their findings in Science Advances, said they designed a serpentine-shaped polymer wrap to create a wrap that can stay in place, while expanding to suit the changing shape of the bladder during urination. It can also hold all the necessary sensors and wiring.
"With the serpentine design built into the structure, we can stretch it to a much bigger geometry," said Cheng. "So, if we stretch that serpentine wrap, which is placed around and against the bladder, it would provide a sufficient force to hold the electronic thread with the sensors in place so that it won't be able to slip off."
The microLEDs, which are an array of micro light-emitting diodes, on the electronic thread are designed to deliver light to the bladder for optogenetic neuromodulation, which modulates the function of the targeted organ.
Cheng said that the device is made from materials that are biologically safe and designed to operate for long-time operation in the body. The wrap does not need sutures or glue to be held in place, which is another benefit, he added.
The team used computational resources from ICDS to investigate various designs of the polymer wrap.
"The computational power is really useful because we needed to design this polymer wrap to different geometries," said Cheng. "In this case, we had two different geometries that we investigated. One is a straight line and the other is the serpentine design. And, of course, we tried a few others, so the computational power allowed us to look at different polymer designs and then single out the best one to use."
In the future, the researchers said the wrap might be re-tooled to help people who have unconscious urination due to overactive bladders. The "smart wrap" implant may also help people with other disease conditions to go from diagnostic confirmation to advanced therapeutic options in clinical medicine.