PR Newswire08.24.20
Stryker launched the Surpass Evolve Flow Diverter following approval by the U.S. Food and Drug Administration. It is the first 64-wire cobalt chromium flow diverter in the U.S. designed to re-direct blood flow and promote aneurysm healing. Surpass Evolve is Stryker's latest innovation in flow diversion and follows the launch of Surpass Streamline in late 2018.
"This device builds on the success of Surpass Streamline, offering a highly optimized and easy to use flow diverter. By increasing the braid angle, the novel 64-wire device delivers excellent flow diversion and a highly flexible implant for enhanced vessel wall contact. The higher mesh density of Surpass Evolve versus traditional 48-wire flow diverters may lead to faster aneurysm occlusion for patients," said Dr. Ajay Wakhloo, a pioneer of flow diversion and the first physician to complete a commercial case in the U.S.
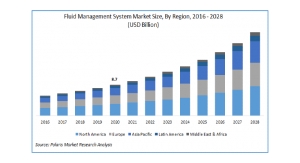
Since its launch in Europe last year, Surpass Evolve has been approved in over 45 countries and more than 1,500 patients have been treated with this life-saving technology. Physicians in these countries have confirmed the device as easier-to-use than its predecessor, offering effortless delivery, predictable deployment and implant opening, and excellent vessel wall apposition. The arrival of Surpass Evolve has been highly anticipated in the U.S. due to these impressive technical and clinical results.
Mark Paul, president of Stryker's Neurovascular division, added, "Surpass Evolve is our fourth PMA approval and second flow diverter approved by the FDA in the last two years. It augments our robust hemorrhagic portfolio and reflects our ongoing commitment to invest in technologies that will drive improved patient outcomes. Stryker is dedicated to working with our customers to bring life-saving technologies to patients suffering from brain aneurysms."
"This device builds on the success of Surpass Streamline, offering a highly optimized and easy to use flow diverter. By increasing the braid angle, the novel 64-wire device delivers excellent flow diversion and a highly flexible implant for enhanced vessel wall contact. The higher mesh density of Surpass Evolve versus traditional 48-wire flow diverters may lead to faster aneurysm occlusion for patients," said Dr. Ajay Wakhloo, a pioneer of flow diversion and the first physician to complete a commercial case in the U.S.
Since its launch in Europe last year, Surpass Evolve has been approved in over 45 countries and more than 1,500 patients have been treated with this life-saving technology. Physicians in these countries have confirmed the device as easier-to-use than its predecessor, offering effortless delivery, predictable deployment and implant opening, and excellent vessel wall apposition. The arrival of Surpass Evolve has been highly anticipated in the U.S. due to these impressive technical and clinical results.
Mark Paul, president of Stryker's Neurovascular division, added, "Surpass Evolve is our fourth PMA approval and second flow diverter approved by the FDA in the last two years. It augments our robust hemorrhagic portfolio and reflects our ongoing commitment to invest in technologies that will drive improved patient outcomes. Stryker is dedicated to working with our customers to bring life-saving technologies to patients suffering from brain aneurysms."