Business Wire08.05.20
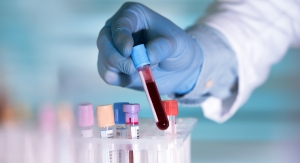
Siemens Healthineers has received FDA Emergency Use Authorization (EUA) for the SARS-CoV-2 IgG (COV2G) antibody test.1 This is the first antibody test authorized with a semi-quantitative detection claim and the fifth antibody test from the company to receive EUA that offers sensitivity and specificity of greater than 99 percent. The COV2G antibody test offers both a positive or negative result for IgG antibodies and reports a numerical result expressed as index value. The test also attained the CE-mark and is now broadly available globally.
A positive or negative result for IgG antibodies provides insight into an individual’s prior exposure to the SARS-CoV-2 virus. Importantly, a semi-quantitative result enables clinicians to gauge the level of IgG antibodies in a patient’s blood sample. With this numerical value, clinicians can establish a baseline and be better equipped to assess changes of an individual’s immune response to the SARS-CoV-2 virus. Comparison of numerical results will help determine how SARS-CoV-2 antibodies develop in an individual and persist over time. A semi-quantitative result is necessary to accurately establish the level of IgG that may be protective, and the Siemens Healthineers’ COV2G assay is well positioned to support this global pursuit.
When an individual is infected with the SARS-CoV-2 virus, unique antibodies will develop at different stages of the infection. Whereas the Siemens Healthineers SARS-CoV-2 Total antibody test detects antibodies to IgM and IgG that are present both early and later during the immune response, the COV2G antibody test specifically detects IgG antibodies that persist, and are the basis for an individual’s longer term immune response. The combination of these tests provides a complete picture of a patient’s serological status for the most accurate results throughout his or her contiuum of care.
“Our high-quality antibody test helps clinicians assess the level of a person‘s immune response, which is an important tool to have at this stage of the pandemic,” said Deepak Nath, Ph.D., president of Laboratory Diagnostics for Siemens Healthineers. “Siemens Healthineers offers a robust portfolio of reliable tests to help support patient care and fight COVID-19.”
Highly accurate antibody test results support key decision-making for individuals and communities. Antibody tests have multiple uses:
Antibody tests from Siemens Healthineers are well-positioned to aid vaccine development efforts. The COV2G antibody test, and all SARS-CoV-2 antibody tests from Siemens Healthineers, detect antibodies to S1RBD. Multiple potential vaccines in development for SARS-CoV-2 include the spike protein, specifically S1RBD—a key protein on the surface of the SARS-CoV-2 virus—within their focus.
Among the considerations for accelerated vaccine approval, according to most recent FDA guidance issued on June 30, validated serology testing in clinical trials may play a pivotal role in assessing surrogate endpoints such as immune response to a vaccine. This requires additional understanding of SARS-CoV-2 immunology and, specifically, vaccine immune responses that might be reasonably likely to predict protection against COVID-19 and post-marketing studies to affirm predicted efficacy. The COV2G test meets or exceeds FDA EUA requirements.6
The COV2G antibody test is available on an expansive installed base of analyzers installed in the U.S. and in countries that accept the CE mark worldwide. This includes the Atellica Solution and ADVIA Centaur XP and XPT families of analyzers. Comparable tests for Siemens Healthineers Dimension Vista and Dimension EXL systems also are being pursued.7
Siemens Healthineers has distinguished itself during the pandemic as a provider of quality antibody tests. For example, in a recent head-to-head evaluation of four commercial antibody tests conducted by Public Health England, in partnership with the University of Oxford, Siemens Healthineers’ COV2T assay was the only test that met both sensitivity and specificity targets.8
To learn more about antibody test offerings from Siemens Healthineers, visit https://www.siemens-healthineers.com/laboratory-diagnostics/assays-by-diseases-conditions/infectious-disease-assays/cov2g-assay.
References
1 This test has not been FDA cleared or approved. This test has been authorized by FDA under an EUA for use by authorized laboratories. This test has been authorized only for detecting the presence of antibodies against SARS-CoV-2, not for any other viruses or pathogens. This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner. Product availability may vary by country and is subject to varying regulatory requirements.
2 https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html Accessed June 21, 2020
3 Bao L, Deng W, Gao H, et al. Reinfection could not occur in SARS-CoV-2 infected rhesus macaques. Posted 2020 May 1. https://doi.org/10.1101/2020.03.13.990226
4 Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Pinto, D. et al. Nature https://doi.org/10.1038/s41586-020-2349-y (2020).
5 Luchsinger LL et al. Serological Analysis of New York City COVID19 Convalescent Plasma Donors. https://doi.org/10.1101/2020.06.08.20124792.This version posted June 9, 2020
6 Positive percent agreement (sensitivity) ≥ 90%; Negative percent agreement (specificity) ≥ 95% https://www.fda.gov/media/137698/download. This version posted June 26, 2020
7 Available under IVD CE Mark. Product availability varies by country.
8 Evaluation of sensitivity and specificity of four commercially available SARS-CoV-2 antibody immunoassays. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/898437/Evaluation__of_sensitivity_and_specificity_of_4_commercially_available_SARS-CoV-2_antibody_immunoassays.pdf. This version posted July 2020
A positive or negative result for IgG antibodies provides insight into an individual’s prior exposure to the SARS-CoV-2 virus. Importantly, a semi-quantitative result enables clinicians to gauge the level of IgG antibodies in a patient’s blood sample. With this numerical value, clinicians can establish a baseline and be better equipped to assess changes of an individual’s immune response to the SARS-CoV-2 virus. Comparison of numerical results will help determine how SARS-CoV-2 antibodies develop in an individual and persist over time. A semi-quantitative result is necessary to accurately establish the level of IgG that may be protective, and the Siemens Healthineers’ COV2G assay is well positioned to support this global pursuit.
When an individual is infected with the SARS-CoV-2 virus, unique antibodies will develop at different stages of the infection. Whereas the Siemens Healthineers SARS-CoV-2 Total antibody test detects antibodies to IgM and IgG that are present both early and later during the immune response, the COV2G antibody test specifically detects IgG antibodies that persist, and are the basis for an individual’s longer term immune response. The combination of these tests provides a complete picture of a patient’s serological status for the most accurate results throughout his or her contiuum of care.
“Our high-quality antibody test helps clinicians assess the level of a person‘s immune response, which is an important tool to have at this stage of the pandemic,” said Deepak Nath, Ph.D., president of Laboratory Diagnostics for Siemens Healthineers. “Siemens Healthineers offers a robust portfolio of reliable tests to help support patient care and fight COVID-19.”
Highly accurate antibody test results support key decision-making for individuals and communities. Antibody tests have multiple uses:
- As an adjunct to PCR tests to aid in clinical assessment2
- To help determine prior exposure to the virus, by detecting antibodies that may neutralize the virus3,4
- To potentially identify donors of convalescent plasma5
- For epidemiological purposes including establishing prevalence of disease in populations
- To potentially help verify effectiveness of vaccines as they become available3,4
Antibody tests from Siemens Healthineers are well-positioned to aid vaccine development efforts. The COV2G antibody test, and all SARS-CoV-2 antibody tests from Siemens Healthineers, detect antibodies to S1RBD. Multiple potential vaccines in development for SARS-CoV-2 include the spike protein, specifically S1RBD—a key protein on the surface of the SARS-CoV-2 virus—within their focus.
Among the considerations for accelerated vaccine approval, according to most recent FDA guidance issued on June 30, validated serology testing in clinical trials may play a pivotal role in assessing surrogate endpoints such as immune response to a vaccine. This requires additional understanding of SARS-CoV-2 immunology and, specifically, vaccine immune responses that might be reasonably likely to predict protection against COVID-19 and post-marketing studies to affirm predicted efficacy. The COV2G test meets or exceeds FDA EUA requirements.6
The COV2G antibody test is available on an expansive installed base of analyzers installed in the U.S. and in countries that accept the CE mark worldwide. This includes the Atellica Solution and ADVIA Centaur XP and XPT families of analyzers. Comparable tests for Siemens Healthineers Dimension Vista and Dimension EXL systems also are being pursued.7
Siemens Healthineers has distinguished itself during the pandemic as a provider of quality antibody tests. For example, in a recent head-to-head evaluation of four commercial antibody tests conducted by Public Health England, in partnership with the University of Oxford, Siemens Healthineers’ COV2T assay was the only test that met both sensitivity and specificity targets.8
To learn more about antibody test offerings from Siemens Healthineers, visit https://www.siemens-healthineers.com/laboratory-diagnostics/assays-by-diseases-conditions/infectious-disease-assays/cov2g-assay.
References
1 This test has not been FDA cleared or approved. This test has been authorized by FDA under an EUA for use by authorized laboratories. This test has been authorized only for detecting the presence of antibodies against SARS-CoV-2, not for any other viruses or pathogens. This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner. Product availability may vary by country and is subject to varying regulatory requirements.
2 https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html Accessed June 21, 2020
3 Bao L, Deng W, Gao H, et al. Reinfection could not occur in SARS-CoV-2 infected rhesus macaques. Posted 2020 May 1. https://doi.org/10.1101/2020.03.13.990226
4 Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Pinto, D. et al. Nature https://doi.org/10.1038/s41586-020-2349-y (2020).
5 Luchsinger LL et al. Serological Analysis of New York City COVID19 Convalescent Plasma Donors. https://doi.org/10.1101/2020.06.08.20124792.This version posted June 9, 2020
6 Positive percent agreement (sensitivity) ≥ 90%; Negative percent agreement (specificity) ≥ 95% https://www.fda.gov/media/137698/download. This version posted June 26, 2020
7 Available under IVD CE Mark. Product availability varies by country.
8 Evaluation of sensitivity and specificity of four commercially available SARS-CoV-2 antibody immunoassays. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/898437/Evaluation__of_sensitivity_and_specificity_of_4_commercially_available_SARS-CoV-2_antibody_immunoassays.pdf. This version posted July 2020