U.S. Food and Drug Administration04.27.20
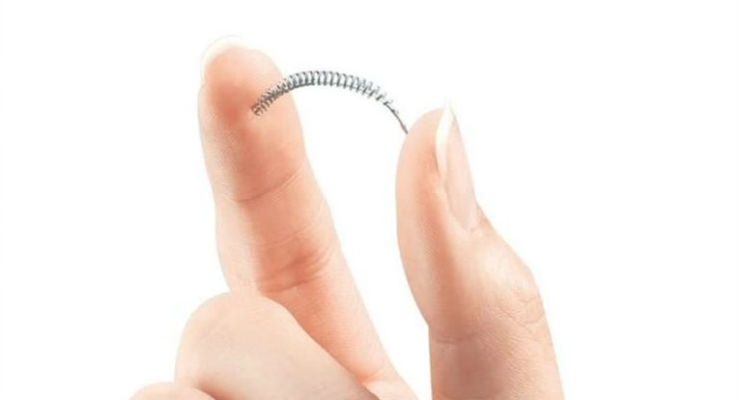
The U.S. Food and Drug Administration issued a letter to Bayer outlining conditions for submitting additional postmarket information to the agency about Essure—a permanent birth control device Bayer sold in the U.S. until 2018. The letter applies to reportable adverse events Bayer is or becomes aware of from social media information received in relation to ongoing litigation regarding Essure.
Because of the anticipated volume and nature of this information, the FDA granted Bayer’s request for a variance from some medical device reporting requirements but imposed conditions for reporting to ensure protection of the public health. Under the conditions, Bayer is required to start providing reportable adverse events to the FDA in July 2020 and continue submitting additional reportable events identified through review of the information on a monthly basis. The FDA will make these events publicly available on its Problems Reported with Essure webpage. The conditions the FDA developed for this particular variance also require Bayer to submit summaries of the reportable events that include information such as the type of event (death, serious injury or malfunction), a description of the reported patient events or device problems, and patient demographics. These summaries will be accessible through the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database. The FDA continues to require that Bayer submit Essure-associated reportable events, outside of the events specifically covered in this variance, in accordance with current medical device reporting requirements.
This variance will expire after one year, unless the FDA grants an extension, at which time, Bayer will be required to follow the regular adverse event reporting requirements for all Essure-associated reportable events.
“Even though the device is no longer being manufactured or distributed, and hasn’t for some time, the FDA continues its engagement with Bayer on postmarket safety monitoring of Essure,” said Terri L. Cornelison, M.D., Ph.D., director of the FDA’s Health of Women Program in the Center for Devices and Radiological Health. “Bayer alerted us to the social media information it received in connection with litigation. We are committed to ensuring that all reportable adverse events identified from this information are submitted to the agency and that they are made publicly available.”
In addition to providing summary information that will be available in MAUDE, Bayer is required to provide quarterly and final analysis reports evaluating the events reported under the variance and how the information compares to other medical device reports (MDRs) for Essure. Bayer will make each analysis report publicly available.
The FDA is also updating the Problems Reported with Essure web page to include updated information on the MDRs the agency has received on the device since its approval. This update includes MDRs received in 2019. While the number of MDRs received in 2019 is the largest received for a single year, due to the continued volume of reports citing litigation and using terms related to device removal, the source and nature of the reports are consistent with recent years.
In addition, Bayer has reported to the agency that all unused Essure devices of which Bayer is aware have been returned to the company, and there should be no devices available for implantation in the U.S. Bayer voluntarily stopped selling and distributing Essure in the U.S. on December 31, 2018. In 2019, the agency reminded health care professionals and facilities to return unused Essure devices to Bayer by the end of that year.
Bayer has also submitted the most recent postmarket (“522”) study report for Essure to the FDA. The FDA will post the interim study results once its review of the data is completed. The FDA believes clinical data from the study will help patients, health care providers and the agency to better understand certain complications that women who have Essure permanent birth control may experience when compared to women who undergo tubal ligation. Findings from the study may serve to inform future FDA actions.
=
Because of the anticipated volume and nature of this information, the FDA granted Bayer’s request for a variance from some medical device reporting requirements but imposed conditions for reporting to ensure protection of the public health. Under the conditions, Bayer is required to start providing reportable adverse events to the FDA in July 2020 and continue submitting additional reportable events identified through review of the information on a monthly basis. The FDA will make these events publicly available on its Problems Reported with Essure webpage. The conditions the FDA developed for this particular variance also require Bayer to submit summaries of the reportable events that include information such as the type of event (death, serious injury or malfunction), a description of the reported patient events or device problems, and patient demographics. These summaries will be accessible through the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database. The FDA continues to require that Bayer submit Essure-associated reportable events, outside of the events specifically covered in this variance, in accordance with current medical device reporting requirements.
This variance will expire after one year, unless the FDA grants an extension, at which time, Bayer will be required to follow the regular adverse event reporting requirements for all Essure-associated reportable events.
“Even though the device is no longer being manufactured or distributed, and hasn’t for some time, the FDA continues its engagement with Bayer on postmarket safety monitoring of Essure,” said Terri L. Cornelison, M.D., Ph.D., director of the FDA’s Health of Women Program in the Center for Devices and Radiological Health. “Bayer alerted us to the social media information it received in connection with litigation. We are committed to ensuring that all reportable adverse events identified from this information are submitted to the agency and that they are made publicly available.”
In addition to providing summary information that will be available in MAUDE, Bayer is required to provide quarterly and final analysis reports evaluating the events reported under the variance and how the information compares to other medical device reports (MDRs) for Essure. Bayer will make each analysis report publicly available.
The FDA is also updating the Problems Reported with Essure web page to include updated information on the MDRs the agency has received on the device since its approval. This update includes MDRs received in 2019. While the number of MDRs received in 2019 is the largest received for a single year, due to the continued volume of reports citing litigation and using terms related to device removal, the source and nature of the reports are consistent with recent years.
In addition, Bayer has reported to the agency that all unused Essure devices of which Bayer is aware have been returned to the company, and there should be no devices available for implantation in the U.S. Bayer voluntarily stopped selling and distributing Essure in the U.S. on December 31, 2018. In 2019, the agency reminded health care professionals and facilities to return unused Essure devices to Bayer by the end of that year.
Bayer has also submitted the most recent postmarket (“522”) study report for Essure to the FDA. The FDA will post the interim study results once its review of the data is completed. The FDA believes clinical data from the study will help patients, health care providers and the agency to better understand certain complications that women who have Essure permanent birth control may experience when compared to women who undergo tubal ligation. Findings from the study may serve to inform future FDA actions.
=