PRNewswire12.03.19
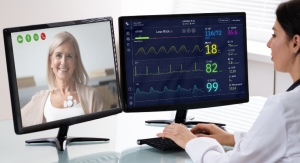
VivaLNK, a provider of connected healthcare solutions, has received Class IIa medical device CE Mark for its multi-vital medical wearable sensor and software development kit (SDK).
Smallest of its Kind
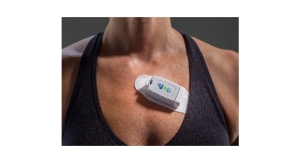
At only 7.5 grams, the reusable patch is the smallest of its kind. Additionally, its reusability factor can increase the economic value of using the patch 10X or more compared to single use devices. Also, the combination of a wearable sensor and an associated SDK enables any application developer to build medical wearable solutions without the need to become experts in hardware and sensor networks.
The multi-vital wearable sensor can generate a continuous stream of ECG rhythm, respiratory rate, heart rate, RR interval, and three-axis accelerometer. Based on VivaLNK's eSkin technology and patient-centric design, the sensor comes in the form of a small bandage sized patch designed to be user friendly, reusable, and well suited for both in-patient and remote patient monitoring (RPM) applications.
"The CE Mark is another major milestone in VivaLNK's pursuit of delivering advanced patient monitoring solutions to market. VivaLNK's unique electronic skin technology offers powerful technical capabilities without compromising user experience," commented Jiang Li, CEO of VivaLNK. "Our aim is to accelerate medical application development by providing the sensor platform so that the industry can rapidly innovate novel solutions while at the same time making it more accessible to patients around the world."
The multi-vital sensor and SDK is part of VivaLNK's Medical Sensor Platform strategy which accelerates medical application innovation by decoupling the sensor hardware, data capture in remote locations, and cloud connectivity from the application so that healthcare solution developers can focus on the algorithms and analytics for their specific domain.
Applications powered by VivaLNK's Medical Sensor Platform include, chemotherapy remote patient monitoring, clinical trial remote patient monitoring, cardiac arrhythmia monitoring, patient infection monitoring, hypertension stress management, and heart failure event detection.
Smallest of its Kind
At only 7.5 grams, the reusable patch is the smallest of its kind. Additionally, its reusability factor can increase the economic value of using the patch 10X or more compared to single use devices. Also, the combination of a wearable sensor and an associated SDK enables any application developer to build medical wearable solutions without the need to become experts in hardware and sensor networks.
The multi-vital wearable sensor can generate a continuous stream of ECG rhythm, respiratory rate, heart rate, RR interval, and three-axis accelerometer. Based on VivaLNK's eSkin technology and patient-centric design, the sensor comes in the form of a small bandage sized patch designed to be user friendly, reusable, and well suited for both in-patient and remote patient monitoring (RPM) applications.
"The CE Mark is another major milestone in VivaLNK's pursuit of delivering advanced patient monitoring solutions to market. VivaLNK's unique electronic skin technology offers powerful technical capabilities without compromising user experience," commented Jiang Li, CEO of VivaLNK. "Our aim is to accelerate medical application development by providing the sensor platform so that the industry can rapidly innovate novel solutions while at the same time making it more accessible to patients around the world."
The multi-vital sensor and SDK is part of VivaLNK's Medical Sensor Platform strategy which accelerates medical application innovation by decoupling the sensor hardware, data capture in remote locations, and cloud connectivity from the application so that healthcare solution developers can focus on the algorithms and analytics for their specific domain.
Applications powered by VivaLNK's Medical Sensor Platform include, chemotherapy remote patient monitoring, clinical trial remote patient monitoring, cardiac arrhythmia monitoring, patient infection monitoring, hypertension stress management, and heart failure event detection.