PR Newswire11.13.19
Laser Associated Sciences (LAS), a medical device company, announced that its blood flow monitoring system, FlowMet-R, received 510(k) clearance from the U.S. Food and Drug Administration (FDA). This clearance allows LAS to market and sell the FlowMet-R. The non-invasive portable device addresses a critical unmet need for vascular specialists who are seeking improved methods to detect and measure treatment efficacy for peripheral artery disease (PAD). The condition, which according to the American Heart Association, affects over 200 million people worldwide, often goes undiagnosed and when left untreated, can result in amputation.
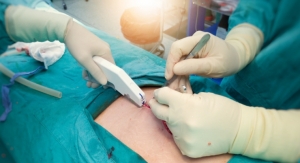
The FlowMet-R is simple for clinicians to use as the device is compact and clips directly onto a patient's toe. The technology includes a small laser diode and a camera the size of a postage stamp to measure blood flow in the digit in real time. This measurement reflects the severity of vascular disease upstream of the digit. Vascular clinicians who use FlowMet-R believe it fills an essential need via intraprocedural monitoring, enabling them to make better decisions about their treatment during surgery.
"By directly measuring limb perfusion during surgeries, physicians can see for the first time whether peripheral blood flow in being improved in real time. This reduces the ambiguity that clinicians currently face in knowing not only if an intervention is effective, but how effective it is. It is inspiring for us to receive such positive feedback from both the medical community and receive clearance from the FDA so quickly," said Sean White, CEO of LAS.
The company also recently completed a clinical study in which FlowMet-R measurements demonstrated improved accuracy in detecting advanced vascular disease compared to standard vascular assessments. "The results were very impactful toward improving patient care, and we look forward to publishing the results shortly," added White.
LAS continues to collaborate with clinicians to develop new technologies that will improve outcomes for patients. The company is also exploring opportunities to build products for home use as well.
Laser Associated Sciences is based in Irvine, Calif. The company's mission is to provide users with critical and impactful information by developing optical biometric sensors and non-invasive medical devices for healthcare applications. In addition to selling and marketing its flagship product, FlowMet-R, LAS is developing additional innovative solutions for the medical community and consumers.
The FlowMet-R is simple for clinicians to use as the device is compact and clips directly onto a patient's toe. The technology includes a small laser diode and a camera the size of a postage stamp to measure blood flow in the digit in real time. This measurement reflects the severity of vascular disease upstream of the digit. Vascular clinicians who use FlowMet-R believe it fills an essential need via intraprocedural monitoring, enabling them to make better decisions about their treatment during surgery.
"By directly measuring limb perfusion during surgeries, physicians can see for the first time whether peripheral blood flow in being improved in real time. This reduces the ambiguity that clinicians currently face in knowing not only if an intervention is effective, but how effective it is. It is inspiring for us to receive such positive feedback from both the medical community and receive clearance from the FDA so quickly," said Sean White, CEO of LAS.
The company also recently completed a clinical study in which FlowMet-R measurements demonstrated improved accuracy in detecting advanced vascular disease compared to standard vascular assessments. "The results were very impactful toward improving patient care, and we look forward to publishing the results shortly," added White.
LAS continues to collaborate with clinicians to develop new technologies that will improve outcomes for patients. The company is also exploring opportunities to build products for home use as well.
Laser Associated Sciences is based in Irvine, Calif. The company's mission is to provide users with critical and impactful information by developing optical biometric sensors and non-invasive medical devices for healthcare applications. In addition to selling and marketing its flagship product, FlowMet-R, LAS is developing additional innovative solutions for the medical community and consumers.