AVS: Science and Technology of Materials, Interfaces, and Processing10.28.19
Modification to the surface of medical devices by using nanoscale beam technology could reduce bacterial attachment to those devices, which could then be implanted inside a patient.
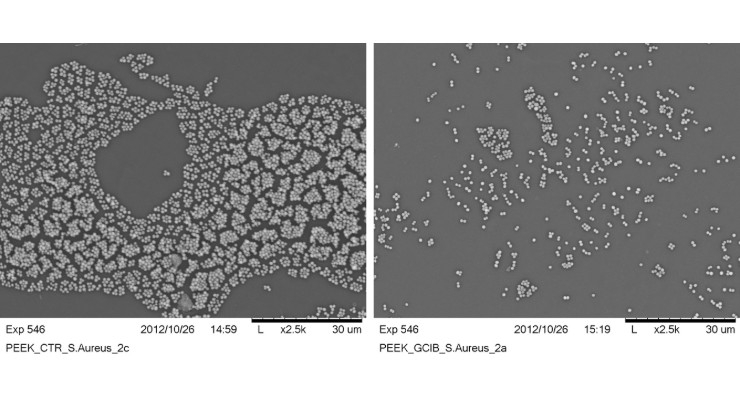
Scientists at Exogenesis Corporation are using accelerated neural atom beam (ANAB) technology, a low-energy accelerated particle beam, to bombard the surface of the medical devices on a very small scale. They found bacterial cells had a decreased ability to attach themselves to the treated surfaces.
At the 66th annual AVS International Symposium and Exhibition in Columbus, Ohio, scientist and Exogenesis Corp. CEO Dimitry Shashkov will discuss how progress has been made to make implantable medical devices safer for patients by using ANAB. The technology can modify a surface to shallow depths of 2 to 3 nanometers, changing its topography and surface energy.
In addition, when a drug coating itself is treated with ANAB, a carbon-rich scaffold is formed on the surface, resulting in a controlled dissolving of the drug.
“We’ve tested a range of polymer materials with regard to bacterial attachment using Staphylococcus aureus and Pseudomonas aeruginosa,” he said. “These are two of the most common pathogens that are linked to hospital-acquired infections and device-associated infections. They represent two fundamentally different types of pathogens, gram-positive and gram-negative bacteria.”
Shashkov believes the same biological reasons that demote bacterial attachment for these two species may also inhibit other pathogens, such as bacterial spores and fungi. With one medical device treated with ANAB receiving approval from the U.S. Food and Drug Administration in 2018 and a second device under review, Shashkov said the practical use of this technology is not far away.
Scientists at Exogenesis Corporation are using accelerated neural atom beam (ANAB) technology, a low-energy accelerated particle beam, to bombard the surface of the medical devices on a very small scale. They found bacterial cells had a decreased ability to attach themselves to the treated surfaces.
At the 66th annual AVS International Symposium and Exhibition in Columbus, Ohio, scientist and Exogenesis Corp. CEO Dimitry Shashkov will discuss how progress has been made to make implantable medical devices safer for patients by using ANAB. The technology can modify a surface to shallow depths of 2 to 3 nanometers, changing its topography and surface energy.
In addition, when a drug coating itself is treated with ANAB, a carbon-rich scaffold is formed on the surface, resulting in a controlled dissolving of the drug.
“We’ve tested a range of polymer materials with regard to bacterial attachment using Staphylococcus aureus and Pseudomonas aeruginosa,” he said. “These are two of the most common pathogens that are linked to hospital-acquired infections and device-associated infections. They represent two fundamentally different types of pathogens, gram-positive and gram-negative bacteria.”
Shashkov believes the same biological reasons that demote bacterial attachment for these two species may also inhibit other pathogens, such as bacterial spores and fungi. With one medical device treated with ANAB receiving approval from the U.S. Food and Drug Administration in 2018 and a second device under review, Shashkov said the practical use of this technology is not far away.