Business Wire10.23.19
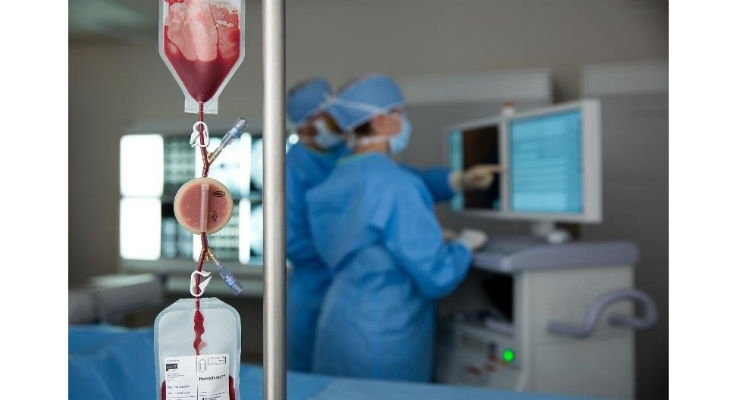
Cook Regentec has enrolled the first patient in an international clinical trial evaluating the HemaTrate Blood Filtration System to treat patients with critical limb ischemia (CLI) due to peripheral arterial disease (PAD). To treat CLI, the HemaTrate system produces an autologous, peripheral blood-derived total nucleated cell (TNC) concentrate for intramuscular injection into the ischemic limb.
“Critical limb ischemia is the final stage of peripheral arterial disease and is associated with major morbidity and mortality despite current medical and surgical treatment,” noted professor Bijan Modarai, the study’s principal investigator, who treated the first enrolled patient at Guy’s and St. Thomas’ Hospital, King’s Health Partners, in London, U.K. “Circulating nucleated cells are thought to stimulate vascular repair and regeneration and are of growing interest as a potential therapeutic option for these patients.”
The HemaTrate CLI (HT-CLI) trial is an international, randomized, controlled, multicenter study that will enroll up to 350 patients. Subjects with Rutherford class 4 or 5 CLI will be randomized to receive a series of three intramuscular injection treatments of TNCs or saline, six weeks apart. The primary endpoint is clinical benefit through 12-month follow-up, defined as freedom from reintervention, major amputation or death. Following the 12-month study period, all patients will be offered TNC treatment for both study and non-study limbs. Patients will be followed for a total of 24 months.
“Patients with PAD-associated CLI who are poor candidates for surgical or endovascular procedures require alternative treatment options that deliver improved long-term outcomes,” said Brad Shirley, business leader at Cook Regentec. “We are excited that patients enrolled in our global CLI trial will begin to receive HemaTrate as treatment for this often-devastating condition.”
Modarai is a principal investigator of the HemaTrate CLI trial and is a paid consultant of Cook Regentec.
Cook Regentec is an Indianapolis-based company that develops the next generation of technologies for the preparation and delivery of biologic and therapeutic agents.
Cook Regentec is part of Cook Group, a family-owned company with headquarters in Bloomington, Indiana. Founded in 1963, Cook Group companies today employ more than 12,000 people around the world. Our diverse business portfolio includes companies working in life sciences, business services, resorts, property management and medical devices.
“Critical limb ischemia is the final stage of peripheral arterial disease and is associated with major morbidity and mortality despite current medical and surgical treatment,” noted professor Bijan Modarai, the study’s principal investigator, who treated the first enrolled patient at Guy’s and St. Thomas’ Hospital, King’s Health Partners, in London, U.K. “Circulating nucleated cells are thought to stimulate vascular repair and regeneration and are of growing interest as a potential therapeutic option for these patients.”
The HemaTrate CLI (HT-CLI) trial is an international, randomized, controlled, multicenter study that will enroll up to 350 patients. Subjects with Rutherford class 4 or 5 CLI will be randomized to receive a series of three intramuscular injection treatments of TNCs or saline, six weeks apart. The primary endpoint is clinical benefit through 12-month follow-up, defined as freedom from reintervention, major amputation or death. Following the 12-month study period, all patients will be offered TNC treatment for both study and non-study limbs. Patients will be followed for a total of 24 months.
“Patients with PAD-associated CLI who are poor candidates for surgical or endovascular procedures require alternative treatment options that deliver improved long-term outcomes,” said Brad Shirley, business leader at Cook Regentec. “We are excited that patients enrolled in our global CLI trial will begin to receive HemaTrate as treatment for this often-devastating condition.”
Modarai is a principal investigator of the HemaTrate CLI trial and is a paid consultant of Cook Regentec.
Cook Regentec is an Indianapolis-based company that develops the next generation of technologies for the preparation and delivery of biologic and therapeutic agents.
Cook Regentec is part of Cook Group, a family-owned company with headquarters in Bloomington, Indiana. Founded in 1963, Cook Group companies today employ more than 12,000 people around the world. Our diverse business portfolio includes companies working in life sciences, business services, resorts, property management and medical devices.