PR Newswire10.21.19
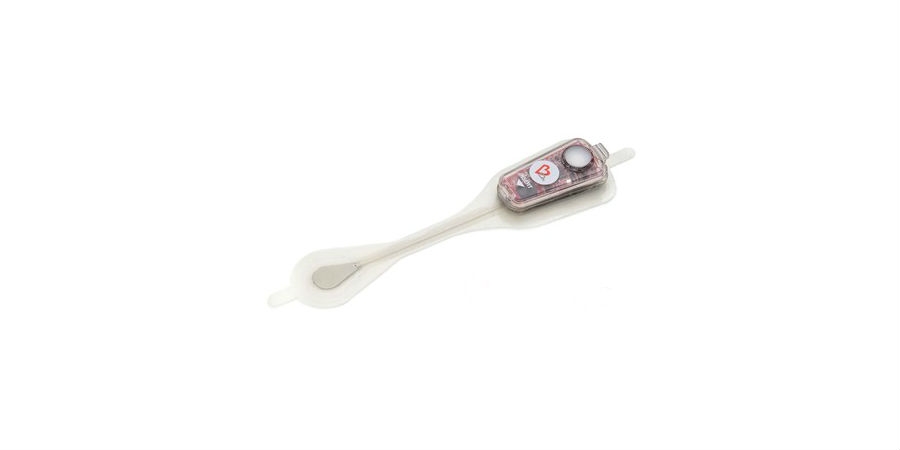
Bardy Diagnostics Inc., a provider of ambulatory cardiac monitoring technologies and custom data solutions, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the 14-Day version of the Carnation Ambulatory Monitor (CAM), the industry's only P-wave centric ambulatory cardiac patch monitor and arrhythmia detection device. Developed to provide clinicians greater flexibility in cardiac monitoring over a longer period, the CAM patch will now be offered in a 14-day extended wear version that utilizes the same innovative P-wave focused technology that powers the existing two-Day and seven-Day CAM product lines.
"Comfortable dermal ECG recordings that focus on the P-wave for up to 14 days carry the potential to minimize use of costly additional rhythm diagnostic tools," said Gust H. Bardy, M.D., founder and CEO of BardyDx. "We are excited to receive clearance from the FDA to enable clinicians the option to monitor longer and anticipate incremental detection of accurate, medically actionable data to improve patient management."
The CAM patch's breakthrough technology reliably detects and records the P-wave, a small amplitude signal of the ECG originating in the atrium that is essential to accurate arrhythmia diagnosis and patient management. Unlike other commercially available patches that include licensed, acquired, or off-the-shelf technologies, the CAM was specially engineered by BardyDx engineers for optimal detection and recording of the P-wave in relation to the rest of the ECG signal to deliver industry leading diagnostic accuracy. The critically acclaimed disruptive technology has been well-received by clinicians, particularly for its ease of use and workflow-friendly design, which allows patients to mail the device back for analysis or the physician office to upload data from a patient's monitor within several minutes upon completion of a study.
The new 14-Day CAM patch enables up to double the duration of P-wave optimized detection and monitoring over the current seven-Day CAM patch, potentially discovering additional, less-frequent arrhythmias. The clinical value of P-wave focused detection was highlighted in the American Heart Journal that published the results of a head-to-head comparison with the iRhythm Zio XT patch. The study, "Comparison of two ambulatory patch ECG monitors: The benefit of the P-wave and signal clarity," (Am Heart J 2018; 203:109-117) concluded that the BardyDx CAM patch identified 40 percent more arrhythmias and resulted in better, more informed clinical decision-making in 41 percent of patients compared to the iRhythm Zio XT patch.
In addition, a preceding study comparing the BardyDx CAM patch and a traditional Holter monitor, entitled "Comparison of diagnostic value using a small, single channel, P-wave centric sternal ECG monitoring patch with a standard 3-lead Holter system over 24 hours," (Am Heart J 2017; 185:65-73) showed a four times increase in arrhythmia detection using the CAM patch, including arrhythmias missed or incorrectly identified using the Holter monitor. The study concluded that the CAM patch offered significantly improved rhythm diagnosis and avoided inaccurate diagnoses made using a Holter monitor.
"Combining the excellence of our P-wave technology with the capability of longer duration monitoring produces an unmatched cardiac monitoring solution," said Ken Nelson, BardyDx chief commercial officer. "We are excited to offer a 14-Day CAM patch that truly provides value for clinicians and patients alike."
Bardy Diagnostics Inc. is an innovator in digital health and remote patient monitoring, with a focus on providing the most diagnostically-accurate and patient-friendly cardiac patch monitors to the industry. The company's CAM patch is a non-invasive, P-wave centric ambulatory cardiac monitor and arrhythmia detection device that is designed to improve patient compliance for adults and children through its lifestyle-enabling design. Designed to be worn comfortably and discreetly, the female-friendly, hourglass-shaped CAM patch is placed on the center of the chest, directly over the heart for optimum ECG signal collection. The proprietary technology of the CAM patch provides optimal detection and clear recording of the often difficult-to-detect P-wave, the signal of the ECG waveform that is essential for accurate arrhythmia diagnosis.
"Comfortable dermal ECG recordings that focus on the P-wave for up to 14 days carry the potential to minimize use of costly additional rhythm diagnostic tools," said Gust H. Bardy, M.D., founder and CEO of BardyDx. "We are excited to receive clearance from the FDA to enable clinicians the option to monitor longer and anticipate incremental detection of accurate, medically actionable data to improve patient management."
The CAM patch's breakthrough technology reliably detects and records the P-wave, a small amplitude signal of the ECG originating in the atrium that is essential to accurate arrhythmia diagnosis and patient management. Unlike other commercially available patches that include licensed, acquired, or off-the-shelf technologies, the CAM was specially engineered by BardyDx engineers for optimal detection and recording of the P-wave in relation to the rest of the ECG signal to deliver industry leading diagnostic accuracy. The critically acclaimed disruptive technology has been well-received by clinicians, particularly for its ease of use and workflow-friendly design, which allows patients to mail the device back for analysis or the physician office to upload data from a patient's monitor within several minutes upon completion of a study.
The new 14-Day CAM patch enables up to double the duration of P-wave optimized detection and monitoring over the current seven-Day CAM patch, potentially discovering additional, less-frequent arrhythmias. The clinical value of P-wave focused detection was highlighted in the American Heart Journal that published the results of a head-to-head comparison with the iRhythm Zio XT patch. The study, "Comparison of two ambulatory patch ECG monitors: The benefit of the P-wave and signal clarity," (Am Heart J 2018; 203:109-117) concluded that the BardyDx CAM patch identified 40 percent more arrhythmias and resulted in better, more informed clinical decision-making in 41 percent of patients compared to the iRhythm Zio XT patch.
In addition, a preceding study comparing the BardyDx CAM patch and a traditional Holter monitor, entitled "Comparison of diagnostic value using a small, single channel, P-wave centric sternal ECG monitoring patch with a standard 3-lead Holter system over 24 hours," (Am Heart J 2017; 185:65-73) showed a four times increase in arrhythmia detection using the CAM patch, including arrhythmias missed or incorrectly identified using the Holter monitor. The study concluded that the CAM patch offered significantly improved rhythm diagnosis and avoided inaccurate diagnoses made using a Holter monitor.
"Combining the excellence of our P-wave technology with the capability of longer duration monitoring produces an unmatched cardiac monitoring solution," said Ken Nelson, BardyDx chief commercial officer. "We are excited to offer a 14-Day CAM patch that truly provides value for clinicians and patients alike."
Bardy Diagnostics Inc. is an innovator in digital health and remote patient monitoring, with a focus on providing the most diagnostically-accurate and patient-friendly cardiac patch monitors to the industry. The company's CAM patch is a non-invasive, P-wave centric ambulatory cardiac monitor and arrhythmia detection device that is designed to improve patient compliance for adults and children through its lifestyle-enabling design. Designed to be worn comfortably and discreetly, the female-friendly, hourglass-shaped CAM patch is placed on the center of the chest, directly over the heart for optimum ECG signal collection. The proprietary technology of the CAM patch provides optimal detection and clear recording of the often difficult-to-detect P-wave, the signal of the ECG waveform that is essential for accurate arrhythmia diagnosis.