Business Wire10.14.19
Apyx Medical Corporation, formerly Bovie Medical Corporation, a maker of medical devices and supplies and the developer of its Helium Plasma Technology, has received U.S. Food and Drug Administration (FDA) 510(k) clearance to market and sell the Apyx Plasma/RF Handpiece, a new addition to the Renuvion product family.
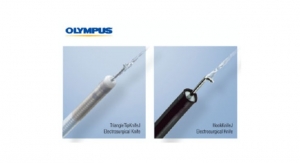
The Apyx Plasma/RF Handpiece was cleared as a sterile, single use electrosurgical (monopolar) device intended to be used in conjunction with compatible generators for the percutaneous delivery of radiofrequency energy and/or helium plasma for cutting, coagulation, and ablation of soft tissue. The Apyx Plasma/RF Handpiece features several important enhancements compared to prior generations, including a smaller diameter instrument shaft (3mm in diameter vs. 5mm in diameter), a bullet-shaped instrument tip that directs the flow of plasma energy from ports on the side of the instrument tip, as opposed to the front of the instrument tip, and a new handle design with improved ergonomics. The handpiece is designed to be used percutaneously.
The handpiece will be available in three configurations: a handpiece with a twin port tip and either a 27cm or 15cm long shaft and a handpiece with a single port tip and a 15cm long shaft. All configurations of the Apyx Plasma/RF Handpiece include features to help minimize unwanted energy application near the incision entry site. Specifically, each handpiece configuration has distance indicators on its tip and will be sold with accompanying epidermal marking templates to help surgeons determine the proximity of the device tip to the entry of the insertion site.
“Apyx Medical Corporation is pleased to announce FDA 510(k) clearance of the Apyx Plasma/RF Handpiece, the latest addition to our Renuvion product family,” said Charlie Goodwin, Chief Executive Officer. “The Apyx Plasma/RF Handpiece is designed specifically for subdermal coagulation and features important enhancements to meet the needs of our surgeon customers in the cosmetic surgery market. During the product development process, we obtained extensive feedback and input from our surgeon customers, as well as the distinguished plastic and cosmetic surgeons on our Medical Advisory Board. We are proud of this robust product development process, and look forward to initiating a limited commercial launch of the Apyx Plasma/RF Handpiece during the fourth quarter of 2019.”
The Apyx Plasma/RF Handpiece was cleared as a sterile, single use electrosurgical (monopolar) device intended to be used in conjunction with compatible generators for the percutaneous delivery of radiofrequency energy and/or helium plasma for cutting, coagulation, and ablation of soft tissue. The Apyx Plasma/RF Handpiece features several important enhancements compared to prior generations, including a smaller diameter instrument shaft (3mm in diameter vs. 5mm in diameter), a bullet-shaped instrument tip that directs the flow of plasma energy from ports on the side of the instrument tip, as opposed to the front of the instrument tip, and a new handle design with improved ergonomics. The handpiece is designed to be used percutaneously.
The handpiece will be available in three configurations: a handpiece with a twin port tip and either a 27cm or 15cm long shaft and a handpiece with a single port tip and a 15cm long shaft. All configurations of the Apyx Plasma/RF Handpiece include features to help minimize unwanted energy application near the incision entry site. Specifically, each handpiece configuration has distance indicators on its tip and will be sold with accompanying epidermal marking templates to help surgeons determine the proximity of the device tip to the entry of the insertion site.
“Apyx Medical Corporation is pleased to announce FDA 510(k) clearance of the Apyx Plasma/RF Handpiece, the latest addition to our Renuvion product family,” said Charlie Goodwin, Chief Executive Officer. “The Apyx Plasma/RF Handpiece is designed specifically for subdermal coagulation and features important enhancements to meet the needs of our surgeon customers in the cosmetic surgery market. During the product development process, we obtained extensive feedback and input from our surgeon customers, as well as the distinguished plastic and cosmetic surgeons on our Medical Advisory Board. We are proud of this robust product development process, and look forward to initiating a limited commercial launch of the Apyx Plasma/RF Handpiece during the fourth quarter of 2019.”