Medtronic10.01.19
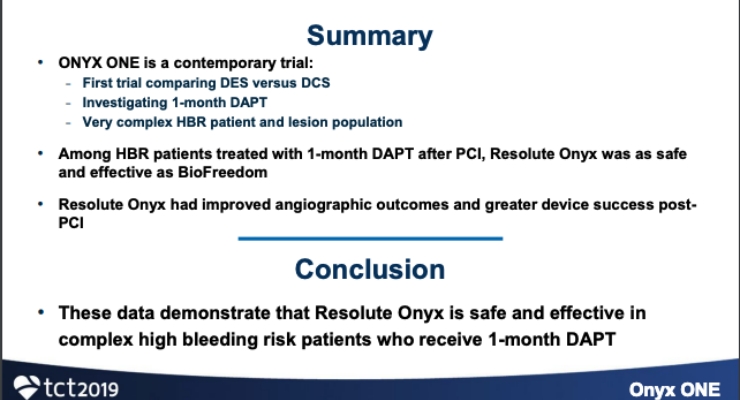
Medtronic plc has shared late-breaking clinical data from the Onyx One Global Study, representing the first prospective, multi-center, randomized study evaluating clinical outcomes between two drug-eluting stents (DES) in nearly 2,000 high-bleeding risk (HBR) patients with one month of dual antiplatelet therapy (DAPT). HBR patients are a complex patient population often excluded from stent and drug trials, make up nearly 40% of all percutaneous coronary intervention (PCI) patients, and may be a group that could benefit from shorter DAPT regimens.
In the study, Resolute Onyx met its primary composite endpoint of cardiac death, myocardial infarction (MI) or stent thrombosis (ST) at one-year showing non-inferiority versus the comparator stent, BioFreedom DCS1 – the only DES discussed by the European Society for Cardiology (ESC) in its guidelines for HBR patients that may need one-month DAPT.
Results were presented during a Late-Breaking Clinical Trial session at the 31st Transcatheter Cardiovascular Therapeutics Conference (TCT), the annual scientific symposium of the Cardiovascular Research Foundation in San Francisco.
“With a growing proportion of PCI patients at a high risk of bleeding, we knew it was critical to generate randomized clinical evidence to evaluate outcomes in this under-represented patient population at increased risk for adverse outcomes,” said Stephan Windecker, M.D., of Bern University Hospital in Switzerland, and principal investigator in the study. “This study provides important advances in evidence for physicians determining the optimal duration of DAPT following PCI among high bleeding risk patients. Data like these are very informative for clinical practice.”
In addition to meeting the primary endpoint, the study showed superior acute performance2 for Resolute Onyx versus BioFreedom, with superior device success of 92.8% versus 89.7%, respectively [p=0.007]. At one year, there was low (2.8%) target lesion revascularization (TLR) for Resolute Onyx versus 4.0% for the comparator stent. Additionally, stent thrombosis (ST) for Resolute Onyx was low (1.3%) versus BioFreedom (2.1%).
A landmark analysis conducted after discontinuation of DAPT at one month showed low event rates for Resolute Onyx3. At one year, the composite endpoint (cardiac death, ST, myocardial infarction [MI]) of this analysis was 7.5% for Resolute Onyx vs. 8.8% for BioFreedom. Additionally, there was significantly lower MI – 4.3% for Resolute Onyx versus 6.8% for BioFreedom (p < 0.01).
The Onyx One Global Study is representative of complex clinical practice of HBR patients including those with anticoagulant use, renal failure, upcoming surgery, and recent blood transfusion. The study protocol did not exclude patients based on disease state or anatomical complexity.
"We are committed to generating clinical evidence to help clinicians better respond to the needs of complex patient populations,” said Dave Moeller, vice president and general manager of the Coronary and Renal Denervation business, which is part of the Cardiac and Vascular Group at Medtronic. “Resolute Onyx DES continues to deliver excellent outcomes for a wide range of patients. With these new data presented today, the evidence provides greater confidence in treating complex patients with Resolute Onyx and a shortened course of DAPT.”
The Resolute Onyx DES encourages fast vessel healing with its proprietary BioLinx polymer, a bio-compatible and non-thrombogenic coating created specifically for use on DES and unique platform design featuring Continuous Sinusoid Technology (CST), which involves forming a single strand of cobalt alloy wire into a sinusoidal wave to construct a stent. The Resolute Onyx DES received CE (Conformité Européene) Mark in September 2014 and FDA approval in April 2017.
To date, more than 20,000 patients have been studied in Medtronic clinical trials that have addressed DAPT duration. The Onyx One Global Study and Onyx One Clear Study make up the Medtronic Onyx One Month DAPT Program that has enrolled approximately 2,700 patients at up to 140 sites worldwide.
In collaboration with leading clinicians, researchers, and scientists worldwide, Medtronic offers the broadest range of innovative medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias. The company strives to offer products and services that deliver clinical and economic value to the healthcare consumers and providers around the world.
References:
1 BioFreedom is not currently approved for use in the United States.
2 Acute performance parameters were not powered or adjusted for multiplicity.
3 Post-hoc landmark analyses were not powered.
In the study, Resolute Onyx met its primary composite endpoint of cardiac death, myocardial infarction (MI) or stent thrombosis (ST) at one-year showing non-inferiority versus the comparator stent, BioFreedom DCS1 – the only DES discussed by the European Society for Cardiology (ESC) in its guidelines for HBR patients that may need one-month DAPT.
Results were presented during a Late-Breaking Clinical Trial session at the 31st Transcatheter Cardiovascular Therapeutics Conference (TCT), the annual scientific symposium of the Cardiovascular Research Foundation in San Francisco.
“With a growing proportion of PCI patients at a high risk of bleeding, we knew it was critical to generate randomized clinical evidence to evaluate outcomes in this under-represented patient population at increased risk for adverse outcomes,” said Stephan Windecker, M.D., of Bern University Hospital in Switzerland, and principal investigator in the study. “This study provides important advances in evidence for physicians determining the optimal duration of DAPT following PCI among high bleeding risk patients. Data like these are very informative for clinical practice.”
In addition to meeting the primary endpoint, the study showed superior acute performance2 for Resolute Onyx versus BioFreedom, with superior device success of 92.8% versus 89.7%, respectively [p=0.007]. At one year, there was low (2.8%) target lesion revascularization (TLR) for Resolute Onyx versus 4.0% for the comparator stent. Additionally, stent thrombosis (ST) for Resolute Onyx was low (1.3%) versus BioFreedom (2.1%).
A landmark analysis conducted after discontinuation of DAPT at one month showed low event rates for Resolute Onyx3. At one year, the composite endpoint (cardiac death, ST, myocardial infarction [MI]) of this analysis was 7.5% for Resolute Onyx vs. 8.8% for BioFreedom. Additionally, there was significantly lower MI – 4.3% for Resolute Onyx versus 6.8% for BioFreedom (p < 0.01).
The Onyx One Global Study is representative of complex clinical practice of HBR patients including those with anticoagulant use, renal failure, upcoming surgery, and recent blood transfusion. The study protocol did not exclude patients based on disease state or anatomical complexity.
"We are committed to generating clinical evidence to help clinicians better respond to the needs of complex patient populations,” said Dave Moeller, vice president and general manager of the Coronary and Renal Denervation business, which is part of the Cardiac and Vascular Group at Medtronic. “Resolute Onyx DES continues to deliver excellent outcomes for a wide range of patients. With these new data presented today, the evidence provides greater confidence in treating complex patients with Resolute Onyx and a shortened course of DAPT.”
The Resolute Onyx DES encourages fast vessel healing with its proprietary BioLinx polymer, a bio-compatible and non-thrombogenic coating created specifically for use on DES and unique platform design featuring Continuous Sinusoid Technology (CST), which involves forming a single strand of cobalt alloy wire into a sinusoidal wave to construct a stent. The Resolute Onyx DES received CE (Conformité Européene) Mark in September 2014 and FDA approval in April 2017.
To date, more than 20,000 patients have been studied in Medtronic clinical trials that have addressed DAPT duration. The Onyx One Global Study and Onyx One Clear Study make up the Medtronic Onyx One Month DAPT Program that has enrolled approximately 2,700 patients at up to 140 sites worldwide.
In collaboration with leading clinicians, researchers, and scientists worldwide, Medtronic offers the broadest range of innovative medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias. The company strives to offer products and services that deliver clinical and economic value to the healthcare consumers and providers around the world.
References:
1 BioFreedom is not currently approved for use in the United States.
2 Acute performance parameters were not powered or adjusted for multiplicity.
3 Post-hoc landmark analyses were not powered.