Business Wire09.12.19
Paragon Biosciences LLC has launched its seventh portfolio company, Qlarity Imaging LLC, which was founded to harness the value of artificial intelligence (AI) to improve medical outcomes.
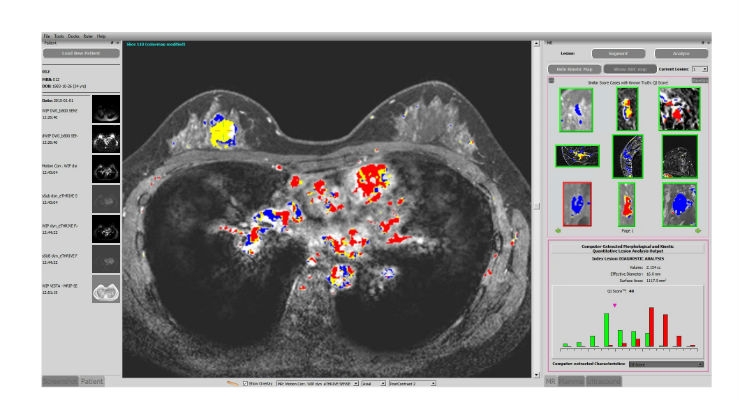
Qlarity Imaging will further develop QuantX, the first-ever U.S. Food and Drug Administration (FDA)-cleared computer-aided breast cancer diagnosis system in radiology. Qlarity plans to expand the diagnostic applications of its AI technology to additional image modalities and medical conditions, with the goal of improving patient care while lowering costs for hospitals and payers.
Qlarity Imaging acquired QuantX, the medical imaging AI system with intuitive displays, advanced analytics, and machine learning, initially developed at the University of Chicago based on research led by Dr. Maryellen L. Giger, and incubated at Quantitative Insights, a startup that had been launched with the support of the University of Chicago’s Polsky Center for Entrepreneurship and Innovation. A clinical study demonstrated the effectiveness of QuantX at helping radiologists interpret cancerous and non-cancerous breast lesions, leading to a 39 percent reduction in missed breast cancers without a reduction in specificity, as well as a 20 percent overall diagnostic improvement. The study led to the FDA clearance of the AI technology for breast cancer.
“By driving innovation across life sciences, Paragon fulfills its mission of improving outcomes for patients with severe medical conditions. So, we’re pleased to help further develop the first FDA-cleared, artificial intelligence-enabled diagnostic software for breast cancer MRIs,” said Paragon Biosciences Chairman and CEO Jeff Aronin. “We are entering an exciting time where advances in supercomputing and machine learning make it possible for artificial intelligence to deliver on its promise for drug discovery, drug development, and diagnostics.”
Paragon’s capabilities and investment in Qlarity Imaging provide the company with the working capital needed to further develop and implement its computer-aided diagnosis system and explore expanded uses of AI-enabled diagnostic tools. Paragon Biosciences advises its portfolio companies on how to leverage AI technology to enhance the diagnostic insight of medical devices, accelerate the pace of drug development, and increase the efficacy of novel therapies.
Qlarity’s AI-enabled image processing and diagnostic algorithms are based on decades of research by professor of Radiology Dr. Maryellen Giger, a pioneer in the field of computer-aided diagnosis. Now an advisor to Qlarity Imaging, Dr. Giger has conducted over 30 years of research in computer-aided diagnosis, including computer vision and machine learning for breast cancer, lung cancer, prostate cancer, lupus, and bone diseases.
“When we looked at how to best commercialize and scale QuantX, the computer-aided diagnostic system originally developed at the University of Chicago, Paragon Biosciences was the perfect partner,” Dr. Giger said. “Paragon is already delivering on its promise, helping Qlarity Imaging to expand its management team, pursue new product opportunities, extend its customer base, and seek additional venture financing.”
With the announcement, Qlarity Imaging becomes the third portfolio company launched by Paragon Biosciences in less than a year and the seventh launched since 2017. Over the last 19 months, Paragon Biosciences and its financial partners have invested and committed over $500 million to help Paragon’s portfolio companies develop innovative therapies and diagnostic tools.
Paragon Biosciences anticipates innovating, investing in, and launching additional portfolio companies this year and next.
Paragon is a life science innovator that invests in, builds, and advises bioscience companies. Its mission is to serve patients living with severe medical conditions which do not yet have adequate treatments. Paragon’s portfolio of independently run bioscience companies focus on: biopharmaceuticals, AI-enabled life science products, and advanced treatments such as cell and gene therapies. We help people live longer, healthier lives.
Qlarity Imaging improves patient care by building AI-driven products that provide clinical insights radiologists do not have access to today. One of Qlarity’s initial products, QuantX Advanced, is the first FDA-cleared, computer-aided diagnosis software for radiology. QuantX integrates images from multiple modalities to assist radiologists in the assessment and characterization of breast abnormalities.

Qlarity Imaging will further develop QuantX, the first-ever U.S. Food and Drug Administration (FDA)-cleared computer-aided breast cancer diagnosis system in radiology. Qlarity plans to expand the diagnostic applications of its AI technology to additional image modalities and medical conditions, with the goal of improving patient care while lowering costs for hospitals and payers.
Qlarity Imaging acquired QuantX, the medical imaging AI system with intuitive displays, advanced analytics, and machine learning, initially developed at the University of Chicago based on research led by Dr. Maryellen L. Giger, and incubated at Quantitative Insights, a startup that had been launched with the support of the University of Chicago’s Polsky Center for Entrepreneurship and Innovation. A clinical study demonstrated the effectiveness of QuantX at helping radiologists interpret cancerous and non-cancerous breast lesions, leading to a 39 percent reduction in missed breast cancers without a reduction in specificity, as well as a 20 percent overall diagnostic improvement. The study led to the FDA clearance of the AI technology for breast cancer.
“By driving innovation across life sciences, Paragon fulfills its mission of improving outcomes for patients with severe medical conditions. So, we’re pleased to help further develop the first FDA-cleared, artificial intelligence-enabled diagnostic software for breast cancer MRIs,” said Paragon Biosciences Chairman and CEO Jeff Aronin. “We are entering an exciting time where advances in supercomputing and machine learning make it possible for artificial intelligence to deliver on its promise for drug discovery, drug development, and diagnostics.”
Paragon’s capabilities and investment in Qlarity Imaging provide the company with the working capital needed to further develop and implement its computer-aided diagnosis system and explore expanded uses of AI-enabled diagnostic tools. Paragon Biosciences advises its portfolio companies on how to leverage AI technology to enhance the diagnostic insight of medical devices, accelerate the pace of drug development, and increase the efficacy of novel therapies.
Qlarity’s AI-enabled image processing and diagnostic algorithms are based on decades of research by professor of Radiology Dr. Maryellen Giger, a pioneer in the field of computer-aided diagnosis. Now an advisor to Qlarity Imaging, Dr. Giger has conducted over 30 years of research in computer-aided diagnosis, including computer vision and machine learning for breast cancer, lung cancer, prostate cancer, lupus, and bone diseases.
“When we looked at how to best commercialize and scale QuantX, the computer-aided diagnostic system originally developed at the University of Chicago, Paragon Biosciences was the perfect partner,” Dr. Giger said. “Paragon is already delivering on its promise, helping Qlarity Imaging to expand its management team, pursue new product opportunities, extend its customer base, and seek additional venture financing.”
With the announcement, Qlarity Imaging becomes the third portfolio company launched by Paragon Biosciences in less than a year and the seventh launched since 2017. Over the last 19 months, Paragon Biosciences and its financial partners have invested and committed over $500 million to help Paragon’s portfolio companies develop innovative therapies and diagnostic tools.
Paragon Biosciences anticipates innovating, investing in, and launching additional portfolio companies this year and next.
Paragon is a life science innovator that invests in, builds, and advises bioscience companies. Its mission is to serve patients living with severe medical conditions which do not yet have adequate treatments. Paragon’s portfolio of independently run bioscience companies focus on: biopharmaceuticals, AI-enabled life science products, and advanced treatments such as cell and gene therapies. We help people live longer, healthier lives.
Qlarity Imaging improves patient care by building AI-driven products that provide clinical insights radiologists do not have access to today. One of Qlarity’s initial products, QuantX Advanced, is the first FDA-cleared, computer-aided diagnosis software for radiology. QuantX integrates images from multiple modalities to assist radiologists in the assessment and characterization of breast abnormalities.