Business Wire08.06.19
Motus GI Holdings Inc.,, a medical technology company dedicated to improving clinical outcomes and enhancing the cost-efficiency of colonoscopy, has appointed Steven M. Bosrock as vice president of Global Marketing & Strategy, and George G. Peters as vice president of Quality & Regulatory Affairs.
“As we work towards the commercial launch of the Pure-Vu GEN2, purposefully building a management team with diverse skillsets and proven expertise has been a key priority for the company. The strategic appointments of Steve and George bring extensive experience in navigating regulatory pathways and launching novel products in significant markets. We continue to be on target for our commercial launch of Pure-Vu GEN2, having just received clearance from the FDA,” commented Tim Moran, CEO of Motus GI.
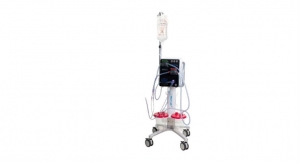
Motus GI recently announced its receipt of Special 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the second-generation Pure-Vu System to help facilitate the cleaning of a poorly prepared colon during the colonoscopy procedure. Pure-Vu GEN2 has been designed to improve the mobility, setup logistics of the system and enhance navigation through the colon, while retaining all the same cleansing functionality as the first generation of the Pure-Vu System.
Bosrock brings to Motus GI significant experience introducing novel products in multiple market opportunities. His track record of building brands, teams, and commercial organizations leading to sustainable growth are key traits he brings to the Motus GI team to drive Pure-Vu into the market. Most recently he served as vice president of Marketing at Torax Medical Incorporated, a privately held medical device company acquired by Johnson & Johnson, that developed and marketed the FDA-approved LINX system to treat gastro-esophageal reflux disease (GERD). Bosrock was responsible for Torax Medical’s U.S. marketing strategy for the launch of LINX. Prior to Torax Medical, Bosrock served as director of Marketing at Synovis Life Technologies, a publicly traded advanced biosurgical device company that was acquired by Baxter International Inc. Additionally, Bosrock held corporate business development and marketing roles at Eli Lilly and Company including responsibility for development and implementation of the U.S. launch of Forteo, a treatment for severe osteoporosis.
“I am excited to join the team at Motus GI at such a pivotal time in the company’s evolution. I believe the Pure-Vu System is a solution that addresses a significant area of unmet need in the colonoscopy market. Over the course of my career, I have built several brands and led the launch of multiple products and I look forward to leveraging my market development experience as we prepare to launch and drive adoption of this important technology,” stated Bosrock.
Peters brings to Motus GI over 25 years of engineering, manufacturing, quality and regulatory experience. Peters’ proven ability to work with regulatory agencies and drive a culture of quality has been a hallmark of his career. Most recently he served as vice president of Quality Systems at Flowonix Medical Inc., a class III PMA electro-mechanical medical device startup company. Prior to that, Peters served as senior director, Quality & Logistics at Metacure (USA), a class III PMA electro-mechanical medical device startup company, as well as a variety of roles in quality assurance and regulatory compliance at Datascope Corp., Interventional Products Division and Cardiac Assist Division.
Peters commented, “As Motus GI continues to lay the foundation for our technology in the hospital market, execution is going to be critical. I look forward to being part of this highly experienced team and applying my experience in regulatory and quality assurance to help position the Pure-Vu System as a potential new standard of care in colonoscopy.”
Motus GI Holdings Inc. is a medical technology company with subsidiaries in the United States and Israel, dedicated to improving clinical outcomes and enhancing the cost-efficiency of colonoscopy. The company’s flagship product is the Pure-Vu System, an FDA-cleared medical device indicated to help facilitate the cleaning of a poorly prepared colon during the colonoscopy procedure. The device integrates with standard and slim colonoscopes to enable safe and rapid cleansing during the procedure while preserving established procedural workflow and techniques. The first generation of the Pure-Vu System has received CE mark approval in Europe. The Pure-Vu System is currently being introduced on a pilot basis in the U.S. market, and the company is planning to initiate a commercial launch focused on the U.S. hospital market in 2019. Challenges with bowel preparation for inpatient colonoscopy represent a significant area of unmet need that directly affects clinical outcomes and increases the cost of care in a market segment that comprises approximately 1.5 million annual procedures in the United States and approximately 4 million annual procedures worldwide. Motus GI believes the Pure-Vu System may improve outcomes and lower costs for hospitals by reducing the time to successful colonoscopy, minimizing delayed and incomplete procedures, and improving the quality of an exam. In clinical studies to date, the Pure-Vu System significantly increased the number of patients with an adequate cleansing level, according to the Boston Bowel Preparation Scale Score, a validated assessment instrument.
“As we work towards the commercial launch of the Pure-Vu GEN2, purposefully building a management team with diverse skillsets and proven expertise has been a key priority for the company. The strategic appointments of Steve and George bring extensive experience in navigating regulatory pathways and launching novel products in significant markets. We continue to be on target for our commercial launch of Pure-Vu GEN2, having just received clearance from the FDA,” commented Tim Moran, CEO of Motus GI.
Motus GI recently announced its receipt of Special 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the second-generation Pure-Vu System to help facilitate the cleaning of a poorly prepared colon during the colonoscopy procedure. Pure-Vu GEN2 has been designed to improve the mobility, setup logistics of the system and enhance navigation through the colon, while retaining all the same cleansing functionality as the first generation of the Pure-Vu System.
Bosrock brings to Motus GI significant experience introducing novel products in multiple market opportunities. His track record of building brands, teams, and commercial organizations leading to sustainable growth are key traits he brings to the Motus GI team to drive Pure-Vu into the market. Most recently he served as vice president of Marketing at Torax Medical Incorporated, a privately held medical device company acquired by Johnson & Johnson, that developed and marketed the FDA-approved LINX system to treat gastro-esophageal reflux disease (GERD). Bosrock was responsible for Torax Medical’s U.S. marketing strategy for the launch of LINX. Prior to Torax Medical, Bosrock served as director of Marketing at Synovis Life Technologies, a publicly traded advanced biosurgical device company that was acquired by Baxter International Inc. Additionally, Bosrock held corporate business development and marketing roles at Eli Lilly and Company including responsibility for development and implementation of the U.S. launch of Forteo, a treatment for severe osteoporosis.
“I am excited to join the team at Motus GI at such a pivotal time in the company’s evolution. I believe the Pure-Vu System is a solution that addresses a significant area of unmet need in the colonoscopy market. Over the course of my career, I have built several brands and led the launch of multiple products and I look forward to leveraging my market development experience as we prepare to launch and drive adoption of this important technology,” stated Bosrock.
Peters brings to Motus GI over 25 years of engineering, manufacturing, quality and regulatory experience. Peters’ proven ability to work with regulatory agencies and drive a culture of quality has been a hallmark of his career. Most recently he served as vice president of Quality Systems at Flowonix Medical Inc., a class III PMA electro-mechanical medical device startup company. Prior to that, Peters served as senior director, Quality & Logistics at Metacure (USA), a class III PMA electro-mechanical medical device startup company, as well as a variety of roles in quality assurance and regulatory compliance at Datascope Corp., Interventional Products Division and Cardiac Assist Division.
Peters commented, “As Motus GI continues to lay the foundation for our technology in the hospital market, execution is going to be critical. I look forward to being part of this highly experienced team and applying my experience in regulatory and quality assurance to help position the Pure-Vu System as a potential new standard of care in colonoscopy.”
Motus GI Holdings Inc. is a medical technology company with subsidiaries in the United States and Israel, dedicated to improving clinical outcomes and enhancing the cost-efficiency of colonoscopy. The company’s flagship product is the Pure-Vu System, an FDA-cleared medical device indicated to help facilitate the cleaning of a poorly prepared colon during the colonoscopy procedure. The device integrates with standard and slim colonoscopes to enable safe and rapid cleansing during the procedure while preserving established procedural workflow and techniques. The first generation of the Pure-Vu System has received CE mark approval in Europe. The Pure-Vu System is currently being introduced on a pilot basis in the U.S. market, and the company is planning to initiate a commercial launch focused on the U.S. hospital market in 2019. Challenges with bowel preparation for inpatient colonoscopy represent a significant area of unmet need that directly affects clinical outcomes and increases the cost of care in a market segment that comprises approximately 1.5 million annual procedures in the United States and approximately 4 million annual procedures worldwide. Motus GI believes the Pure-Vu System may improve outcomes and lower costs for hospitals by reducing the time to successful colonoscopy, minimizing delayed and incomplete procedures, and improving the quality of an exam. In clinical studies to date, the Pure-Vu System significantly increased the number of patients with an adequate cleansing level, according to the Boston Bowel Preparation Scale Score, a validated assessment instrument.