Business Wire07.31.19
The University of Chicago Medicine's Comprehensive Hypertension Center is participating in a U.S. Food and Drug Administration (FDA)-approved pivotal trial of a novel catheter-based, non-surgical procedure to treat patients with drug-resistant hypertension. This study, called CALM-2 (Controlling And Lowering blood pressure with MobiusHD), continues the study of the investigational MobiusHD device which was evaluated in an earlier proof-of-concept CALM-FIM which showed significant reductions in blood pressure through six months.
The new, larger, multi-center CALM-2 clinical trial has been designed to assess the safety and effectiveness of this unique device as a possible solution for patients whose blood pressure is not controlled with prescribed medications.
The research team at the Comprehensive Hypertension Center, led by world-renowned hypertension expert, professor of medicine and Center Director George Bakris, M.D., specializes in the assessment and treatment of hard-to-treat hypertension. This includes the assessment of studies examining innovations in resistant hypertension.
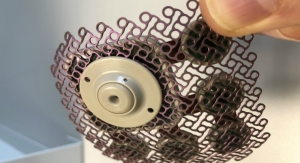
Specialized stretch-sensitive nerves called baroreceptors are located in the walls of the carotid arteries and play an essential role in the body’s natural blood pressure regulation. The MobiusHD system is used in the first minimally invasive procedure to utilize the baroreceptor mechanism to address uncontrolled hypertension.
“Patients with high blood pressure who are non-responsive to multiple medications have very few treatment options,” said Bakris. “Based on our experience, device-based approaches targeting baroreceptors as a therapeutic option have promise. The initial results using the MobiusHD implant to reshape the carotid sinus and stretch baroreceptors to modulate the body’s natural blood pressure regulation are impressive, and we are excited to participate in the CALM-2 clinical trial to fully evaluate the safety and effectiveness of this new treatment.”
The CALM-2 clinical trial, sponsored by Vascular Dynamics Inc., is targeting up to 300 drug-resistant hypertension patients at select medical centers around the United States and in the United Kingdom and Europe, including UChicago Medicine.
Vascular Dynamics develops minimally invasive platform technologies to offer treatment options for patients at risk of life-threatening cardiovascular events and underserved by conventional treatments. Focused initially on uncontrolled hypertension, VDI was approved to participate in the FDA's Breakthrough Devices Program (formerly known as the Expedited Access Pathway (EAP) program). The MobiusHD implant system has received a CE Mark for the treatment of hypertension in the European Union; however, the MobiusHD system is not commercially available in the United States.
In the United States, the MobiusHD device is limited by law to investigational use only.
The new, larger, multi-center CALM-2 clinical trial has been designed to assess the safety and effectiveness of this unique device as a possible solution for patients whose blood pressure is not controlled with prescribed medications.
The research team at the Comprehensive Hypertension Center, led by world-renowned hypertension expert, professor of medicine and Center Director George Bakris, M.D., specializes in the assessment and treatment of hard-to-treat hypertension. This includes the assessment of studies examining innovations in resistant hypertension.
Specialized stretch-sensitive nerves called baroreceptors are located in the walls of the carotid arteries and play an essential role in the body’s natural blood pressure regulation. The MobiusHD system is used in the first minimally invasive procedure to utilize the baroreceptor mechanism to address uncontrolled hypertension.
“Patients with high blood pressure who are non-responsive to multiple medications have very few treatment options,” said Bakris. “Based on our experience, device-based approaches targeting baroreceptors as a therapeutic option have promise. The initial results using the MobiusHD implant to reshape the carotid sinus and stretch baroreceptors to modulate the body’s natural blood pressure regulation are impressive, and we are excited to participate in the CALM-2 clinical trial to fully evaluate the safety and effectiveness of this new treatment.”
The CALM-2 clinical trial, sponsored by Vascular Dynamics Inc., is targeting up to 300 drug-resistant hypertension patients at select medical centers around the United States and in the United Kingdom and Europe, including UChicago Medicine.
Vascular Dynamics develops minimally invasive platform technologies to offer treatment options for patients at risk of life-threatening cardiovascular events and underserved by conventional treatments. Focused initially on uncontrolled hypertension, VDI was approved to participate in the FDA's Breakthrough Devices Program (formerly known as the Expedited Access Pathway (EAP) program). The MobiusHD implant system has received a CE Mark for the treatment of hypertension in the European Union; however, the MobiusHD system is not commercially available in the United States.
In the United States, the MobiusHD device is limited by law to investigational use only.