Rob Matheson, MIT News Office07.23.19
A novel sensor designed by MIT researchers could dramatically accelerate the process of diagnosing sepsis, a leading cause of death in U.S. hospitals that kills nearly 250,000 patients annually.
Sepsis occurs when the body’s immune response to infection triggers an inflammation chain reaction throughout the body, causing high heart rate, high fever, shortness of breath, and other issues. If left unchecked, it can lead to septic shock, where blood pressure falls and organs shut down. To diagnose sepsis, doctors traditionally rely on various diagnostic tools, including vital signs, blood tests, and other imaging and lab tests.
In recent years, researchers have found protein biomarkers in the blood that are early indicators of sepsis. One promising candidate is interleukin-6 (IL-6), a protein produced in response to inflammation. In sepsis patients, IL-6 levels can rise hours before other symptoms begin to show. But even at these elevated levels, the concentration of this protein in the blood is too low overall for traditional assay devices to detect it quickly.
In a paper being presented this week at the Engineering in Medicine and Biology Conference, MIT researchers describe a microfluidics-based system that automatically detects clinically significant levels of IL-6 for sepsis diagnosis in about 25 minutes, using less than a finger prick of blood.
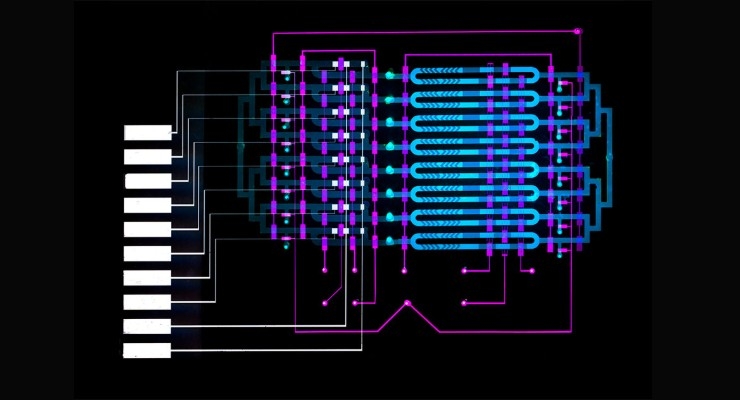
In one microfluidic channel, microbeads laced with antibodies mix with a blood sample to capture the IL-6 biomarker. In another channel, only beads containing the biomarker attach to an electrode. Running voltage through the electrode produces an electrical signal for each biomarker-laced bead, which is then converted into the biomarker concentration level.
“For an acute disease, such as sepsis, which progresses very rapidly and can be life-threatening, it’s helpful to have a system that rapidly measures these nonabundant biomarkers,” said first author Dan Wu, a PhD student in the Department of Mechanical Engineering. “You can also frequently monitor the disease as it progresses.”
Joining Wu on the paper is Joel Voldman, a professor and associate head of the Department of Electrical Engineering and Computer Science, co-director of the Medical Electronic Device Realization Center, and a principal investigator in the Research Laboratory of Electronics and the Microsystems Technology Laboratories.
Integrated, Automated Design
Traditional assaid that detect protein biomarkers are bulky, expensive machines relegated to labs that require about a milliliter of blood and produce results in hours. In recent years, portable “point-of-care” systems have been developed that use microliters of blood to get similar results in about 30 minutes.
But point-of-care systems can be very expensive since most use pricey optical components to detect the biomarkers. They also capture only a small number of proteins, many of which are among the more abundant ones in blood. Any efforts to decrease the price, shrink down components, or increase protein ranges negatively impacts their sensitivity.
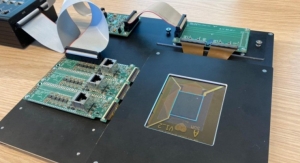
In their work, the researchers wanted to shrink components of the magnetic-bead-based assay, which is often used in labs, onto an automated microfluidics device that’s roughly several square centimeters. That required manipulating beads in micron-sized channels and fabricating a device in the Microsystems Technology Laboratory that automated the movement of fluids.
The beads are coated with an antibody that attracts IL-6, as well as a catalyzing enzyme called horseradish peroxidase. The beads and blood sample are injected into the device, entering into an “analyte-capture zone,” which is basically a loop. Along the loop is a peristaltic pump—commonly used for controlling liquids—with valves automatically controlled by an external circuit. Opening and closing the valves in specific sequences circulates the blood and beads to mix together. After about 10 minutes, the IL-6 proteins have bound to the antibodies on the beads.
Automatically reconfiguring the valves at that time forces the mixture into a smaller loop, called the “detection zone,” where they stay trapped. A tiny magnet collects the beads for a brief wash before releasing them around the loop. After about 10 minutes, many beads have stuck on an electrode coated with a separate antibody that attracts IL-6. At that time, a solution flows into the loop and washes the untethered beads, while the ones with IL-6 protein remain on the electrode.
The solution carries a specific molecule that reacts to the horseradish enzyme to create a compound that responds to electricity. When a voltage is applied to the solution, each remaining bead creates a small current. A common chemistry technique called “amperometry” converts that current into a readable signal. The device counts the signals and calculates the concentration of IL-6.
“On their end, doctors just load in a blood sample using a pipette. Then, they press a button and 25 minutes later they know the IL-6 concentration,” Wu said.
The device uses about 5 microliters of blood, which is about a quarter the volume of blood drawn from a finger prick and a fraction of the 100 microliters required to detect protein biomarkers in lab-based assaid. The device captures IL-6 concentrations as low as 16 picograms per milliliter, which is below the concentrations that signal sepsis, meaning the device is sensitive enough to provide clinically relevant detection.
A General Platform
The current design has eight separate microfluidics channels to measure as many different biomarkers or blood samples in parallel. Different antibodies and enzymes can be used in separate channels to detect different biomarkers, or different antibodies can be used in the same channel to detect several biomarkers simultaneously.
Next, the researchers plan to create a panel of important sepsis biomarkers for the device to capture, including interleukin-6, interleukin-8, C-reactive protein, and procalcitonin. But there’s really no limit to how many different biomarkers the device can measure, for any disease, Wu said. Notably, more than 200 protein biomarkers for various diseases and conditions have been approved by the U.S. Food and Drug Administration.
“This is a very general platform,” Wu said. “If you want to increase the device’s physical footprint, you can scale up and design more channels to detect as many biomarkers as you want.”
The work was funded by Analog Devices, Maxim Integrated, and the Novartis Institutes of Biomedical Research.
Sepsis occurs when the body’s immune response to infection triggers an inflammation chain reaction throughout the body, causing high heart rate, high fever, shortness of breath, and other issues. If left unchecked, it can lead to septic shock, where blood pressure falls and organs shut down. To diagnose sepsis, doctors traditionally rely on various diagnostic tools, including vital signs, blood tests, and other imaging and lab tests.
In recent years, researchers have found protein biomarkers in the blood that are early indicators of sepsis. One promising candidate is interleukin-6 (IL-6), a protein produced in response to inflammation. In sepsis patients, IL-6 levels can rise hours before other symptoms begin to show. But even at these elevated levels, the concentration of this protein in the blood is too low overall for traditional assay devices to detect it quickly.
In a paper being presented this week at the Engineering in Medicine and Biology Conference, MIT researchers describe a microfluidics-based system that automatically detects clinically significant levels of IL-6 for sepsis diagnosis in about 25 minutes, using less than a finger prick of blood.
In one microfluidic channel, microbeads laced with antibodies mix with a blood sample to capture the IL-6 biomarker. In another channel, only beads containing the biomarker attach to an electrode. Running voltage through the electrode produces an electrical signal for each biomarker-laced bead, which is then converted into the biomarker concentration level.
“For an acute disease, such as sepsis, which progresses very rapidly and can be life-threatening, it’s helpful to have a system that rapidly measures these nonabundant biomarkers,” said first author Dan Wu, a PhD student in the Department of Mechanical Engineering. “You can also frequently monitor the disease as it progresses.”
Joining Wu on the paper is Joel Voldman, a professor and associate head of the Department of Electrical Engineering and Computer Science, co-director of the Medical Electronic Device Realization Center, and a principal investigator in the Research Laboratory of Electronics and the Microsystems Technology Laboratories.
Integrated, Automated Design
Traditional assaid that detect protein biomarkers are bulky, expensive machines relegated to labs that require about a milliliter of blood and produce results in hours. In recent years, portable “point-of-care” systems have been developed that use microliters of blood to get similar results in about 30 minutes.
But point-of-care systems can be very expensive since most use pricey optical components to detect the biomarkers. They also capture only a small number of proteins, many of which are among the more abundant ones in blood. Any efforts to decrease the price, shrink down components, or increase protein ranges negatively impacts their sensitivity.
In their work, the researchers wanted to shrink components of the magnetic-bead-based assay, which is often used in labs, onto an automated microfluidics device that’s roughly several square centimeters. That required manipulating beads in micron-sized channels and fabricating a device in the Microsystems Technology Laboratory that automated the movement of fluids.
The beads are coated with an antibody that attracts IL-6, as well as a catalyzing enzyme called horseradish peroxidase. The beads and blood sample are injected into the device, entering into an “analyte-capture zone,” which is basically a loop. Along the loop is a peristaltic pump—commonly used for controlling liquids—with valves automatically controlled by an external circuit. Opening and closing the valves in specific sequences circulates the blood and beads to mix together. After about 10 minutes, the IL-6 proteins have bound to the antibodies on the beads.
Automatically reconfiguring the valves at that time forces the mixture into a smaller loop, called the “detection zone,” where they stay trapped. A tiny magnet collects the beads for a brief wash before releasing them around the loop. After about 10 minutes, many beads have stuck on an electrode coated with a separate antibody that attracts IL-6. At that time, a solution flows into the loop and washes the untethered beads, while the ones with IL-6 protein remain on the electrode.
The solution carries a specific molecule that reacts to the horseradish enzyme to create a compound that responds to electricity. When a voltage is applied to the solution, each remaining bead creates a small current. A common chemistry technique called “amperometry” converts that current into a readable signal. The device counts the signals and calculates the concentration of IL-6.
“On their end, doctors just load in a blood sample using a pipette. Then, they press a button and 25 minutes later they know the IL-6 concentration,” Wu said.
The device uses about 5 microliters of blood, which is about a quarter the volume of blood drawn from a finger prick and a fraction of the 100 microliters required to detect protein biomarkers in lab-based assaid. The device captures IL-6 concentrations as low as 16 picograms per milliliter, which is below the concentrations that signal sepsis, meaning the device is sensitive enough to provide clinically relevant detection.
A General Platform
The current design has eight separate microfluidics channels to measure as many different biomarkers or blood samples in parallel. Different antibodies and enzymes can be used in separate channels to detect different biomarkers, or different antibodies can be used in the same channel to detect several biomarkers simultaneously.
Next, the researchers plan to create a panel of important sepsis biomarkers for the device to capture, including interleukin-6, interleukin-8, C-reactive protein, and procalcitonin. But there’s really no limit to how many different biomarkers the device can measure, for any disease, Wu said. Notably, more than 200 protein biomarkers for various diseases and conditions have been approved by the U.S. Food and Drug Administration.
“This is a very general platform,” Wu said. “If you want to increase the device’s physical footprint, you can scale up and design more channels to detect as many biomarkers as you want.”
The work was funded by Analog Devices, Maxim Integrated, and the Novartis Institutes of Biomedical Research.