Business Wire05.29.19
Fist Assist Devices LLC, a medical device startup based in Silicon Valley, Calif., has announced the approval of FACT (Fist Assist Clinical Trial) by the University of Chicago Medical Center Institutional Review Board (IRB). This research will evaluate the effects of using the Fist Assist device to develop superficial veins prior to arteriovenous fistula (AVF) creation for patients with end-stage renal disease (ESRD) by either surgery or new endovascular procedures.
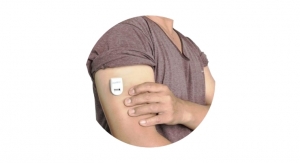
The Fist Assist device is the only wearable, patent-protected intermittent compression device that supports vein dilation for patients with ESRD. The non-invasive device applies intermittent pressure to a specific superficial arm vein to help dilate the vein prior to surgical or endovascular fistula placement. As extensively studied, larger veins before fistula creation can ensure that the fistula reaches optimal size without delays or extra procedures, ultimately reducing costs for both patients and dialysis providers.
“This truly is an exciting time for our company. Partnering with Dr. Mary Hammes, associate professor in the Department of Nephrology at the University of Chicago, and her colleagues is a great opportunity to conduct this pioneering research at the Medical Center where the concept for the device was formulated more than 30 years ago,” said Tej Singh M.D., CEO and founder of Fist Assist Devices LLC. “We are confident that Dr. Hammes and her team will conduct a high-quality study that will serve as the foundation for our FDA de novo submission. This non-significant risk trial will be carefully watched by many, including Fist Assist Devices LLC’s newly created Medical Advisory Board.”
Today, renal failure affects about 20 million people in the United States, and it is expected to affect 4.9 million patients globally by 2025,1 and many of these cases will progress to ESRD. Currently, 437,000 patients in the United States receive hemodialysis treatment for ESRD, and an additional 150,000 patients are expected to be added to this number annually.2 These statistics demonstrate the urgent need to find more effective methods to promote vein dilation and develop cost-effective, non-invasive devices to help AVF mature and prevent renal failure.
“We are delighted to conduct the FACT trial and work with the Fist Assist device,” said Dr. Mary Hammes, associate professor of Medicine, director, Chronic Hemodialysis at the University of Chicago. “The Fist Assist device is a groundbreaking device for patients with end-stage renal diseases. We hope our study demonstrates the significant benefit of the device to patients, providers and everyone affected by ESRD.”
Fist Assist Devices LLC is a privately held company located in Silicon Valley, Calif., that has developed the patent-protected Fist Assist technology over the past 30 years. Fist Assist Devices LLC develops and markets an intermittent, external wearable pneumatic compression to increase arm vein enhancement and AV fistula dilation. The device is currently approved for sale in India, but is not yet commercially available in Europe or the United States.
References
1 Fresenius Medical Care, www.freseniusmedicalcare.com/en/investors/at-a-glance/outlook/
2 Centers for Disease Control and Prevention, www.cdc.gov/kidneydisease/publications-resources/2019-national-facts.html#calculation
The Fist Assist device is the only wearable, patent-protected intermittent compression device that supports vein dilation for patients with ESRD. The non-invasive device applies intermittent pressure to a specific superficial arm vein to help dilate the vein prior to surgical or endovascular fistula placement. As extensively studied, larger veins before fistula creation can ensure that the fistula reaches optimal size without delays or extra procedures, ultimately reducing costs for both patients and dialysis providers.
“This truly is an exciting time for our company. Partnering with Dr. Mary Hammes, associate professor in the Department of Nephrology at the University of Chicago, and her colleagues is a great opportunity to conduct this pioneering research at the Medical Center where the concept for the device was formulated more than 30 years ago,” said Tej Singh M.D., CEO and founder of Fist Assist Devices LLC. “We are confident that Dr. Hammes and her team will conduct a high-quality study that will serve as the foundation for our FDA de novo submission. This non-significant risk trial will be carefully watched by many, including Fist Assist Devices LLC’s newly created Medical Advisory Board.”
Today, renal failure affects about 20 million people in the United States, and it is expected to affect 4.9 million patients globally by 2025,1 and many of these cases will progress to ESRD. Currently, 437,000 patients in the United States receive hemodialysis treatment for ESRD, and an additional 150,000 patients are expected to be added to this number annually.2 These statistics demonstrate the urgent need to find more effective methods to promote vein dilation and develop cost-effective, non-invasive devices to help AVF mature and prevent renal failure.
“We are delighted to conduct the FACT trial and work with the Fist Assist device,” said Dr. Mary Hammes, associate professor of Medicine, director, Chronic Hemodialysis at the University of Chicago. “The Fist Assist device is a groundbreaking device for patients with end-stage renal diseases. We hope our study demonstrates the significant benefit of the device to patients, providers and everyone affected by ESRD.”
Fist Assist Devices LLC is a privately held company located in Silicon Valley, Calif., that has developed the patent-protected Fist Assist technology over the past 30 years. Fist Assist Devices LLC develops and markets an intermittent, external wearable pneumatic compression to increase arm vein enhancement and AV fistula dilation. The device is currently approved for sale in India, but is not yet commercially available in Europe or the United States.
References
1 Fresenius Medical Care, www.freseniusmedicalcare.com/en/investors/at-a-glance/outlook/
2 Centers for Disease Control and Prevention, www.cdc.gov/kidneydisease/publications-resources/2019-national-facts.html#calculation