U.S. Department of Justice04.10.19
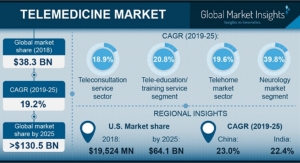
One of the largest healthcare fraud schemes investigated by the FBI and the U.S. Department of Health and Human Services Office of the Inspector General (HHS-OIG) and prosecuted by the Department of Justice resulted in charges against 24 defendants, including the CEOs, COOs and others associated with five telemedicine companies, the owners of dozens of durable medical equipment (DME) companies and three licensed medical professionals, for their alleged participation in healthcare fraud schemes involving more than $1.2 billion in loss, as well as the execution of over 80 search warrants in 17 federal districts. In addition, the Center for Medicare Services, Center for Program Integrity (CMS/CPI) announced that it took adverse administrative action against 130 DME companies that had submitted over $1.7 billion in claims and were paid over $900 million.
Assistant Attorney General Brian A. Benczkowski of the Justice Department’s Criminal Division, U.S. Attorney Sherri A. Lydon of the District of South Carolina, U.S. Attorney Craig Carpenito of the District of New Jersey, U.S. Attorney Maria Chapa Lopez of the Middle District of Florida, Assistant Director Robert Johnson of the FBI’s Criminal Investigative Division, Deputy Inspector General for Investigations Gary Cantrell of the U.S. Department of Health and Human Services Office of Inspector General (HHS-OIG), Chief Don Fort of the IRS Criminal Investigation (CI) and Deputy Administrator and Director of CPI Alec Alexander of the CMS/CPI made the announcement.
The enforcement actions were led and coordinated by the Health Care Fraud Unit of the Criminal Division’s Fraud Section in conjunction with its Medicare Fraud Strike Force (MFSF), as well as the U.S. Attorney’s Offices for the Districts of South Carolina, New Jersey and the Middle District of Florida. The MFSF is a partnership among the Criminal Division, U.S. Attorney’s Offices, the FBI and HHS-OIG. In addition, IRS-CI and other federal law enforcement agencies participated in the operation.
The charges announced target an alleged scheme involving the payment of illegal kickbacks and bribes by DME companies in exchange for the referral of Medicare beneficiaries by medical professionals working with fraudulent telemedicine companies for back, shoulder, wrist and knee braces that are medically unnecessary. Some of the defendants allegedly controlled an international telemarketing network that lured over hundreds of thousands of elderly and/or disabled patients into a criminal scheme that crossed borders, involving call centers in the Philippines and throughout Latin America. The defendants allegedly paid doctors to prescribe DME either without any patient interaction or with only a brief telephonic conversation with patients they had never met or seen. The proceeds of the fraudulent scheme were allegedly laundered through international shell corporations and used to purchase exotic automobiles, yachts and luxury real estate in the United States and abroad.
“These defendants—who range from corporate executives to medical professionals—allegedly participated in an expansive and sophisticated fraud to exploit telemedicine technology meant for patients otherwise unable to access healthcare,” said Assistant Attorney General Benczkowski. “This Department of Justice will not tolerate medical professionals and executives who look to line their pockets by cheating our healthcare programs. I commend the Criminal Division prosecutors and our partners from U.S. Attorney’s Offices and law enforcement agencies across the country for their unrelenting efforts to stop this alleged fraud before more money was stolen from American taxpayers.”
“Simply put, the law applies equally to all in South Carolina,” said U.S. Attorney Sherri Lydon. “The same spoon that serves indictments on drug dealers, felons in possession of firearms, and corrupt officials will also feed those companies and individuals who engage in Medicare fraud. White collar crime is not victimless. All taxpayers will endure the rising cost of healthcare premiums and out-of-pocket costs as a result of fraud on our Medicare system. I am honored to stand with our partners at the FBI, HHS-OIG, and IRS-CI, who led this outstanding and nationally significant investigation from right here in South Carolina.”
“The indictments we are unsealing charge the defendants with running a complex, multilayered scheme to defraud our Medicare system and avoid detection by government regulators,” said U.S. Attorney Craig Carpenito. “The defendants took advantage of unwitting patients who were simply trying to get relief from their health concerns. Instead, the defendants preyed upon their weakened state and pushed millions of dollars’ worth of unnecessary medical devices, which Medicare paid for, and then set up an elaborate system for laundering their ill-gotten proceeds. We are proud to join our law enforcement partners in New Jersey and around the country to put a stop to this unscrupulous criminal activity.”
“Protecting the integrity of America’s healthcare programs is necessary to ensure that our citizens receive the care they have paid for and deserve,” said U.S. Attorney Chapa Lopez. “The mammoth coordination and cooperation demonstrated among the various offices, districts, and agencies involved in this case leaves no doubt. We will leverage the full weight of our resources to combat fraud and abuse, wherever it is found.”
“One of the largest healthcare fraud schemes in U.S. history came to an end thanks to close collaboration and coordination between the FBI and partners including HHS-OIG and IRS-CI,” said FBI Assistant Director Robert Johnson. “Healthcare fraud causes billions of dollars in losses, it deprives real patients of the critical healthcare services they need, and it can endanger the lives of real patients so individuals like those arrested can profit from their criminal activity. Through the coordinated national effort, we put an end to this egregious and costly healthcare fraud scheme, and the public can rest assured the FBI will continue to make healthcare fraud investigations a top priority.”
“Our law enforcement officers are focused on preventing and uprooting healthcare fraud schemes like those alleged,” said Deputy Inspector General for Investigations Gary Cantrell. “These schemes divert money from taxpayer-funded federal healthcare programs into the hands of criminals. Working closely with our law enforcement partners, our agency will continue to investigate and disrupt attempts to undermine Medicare and target beneficiaries.”
“The breadth of this nationwide conspiracy should be frightening to all who rely on some form of healthcare,” said IRS-CI Chief Don Fort. “The conspiracy described in this indictment was not perpetrated by one individual. Rather, it details broad corruption, massive amounts of greed, and systemic flaws in our healthcare system that were exploited by the defendants. We all suffer when schemes like this go undiscovered and I’m proud of the work our agents did in working with our partners to uncover this complex scheme.”
“The Centers for Medicare & Medicaid Services (CMS) Center for Program Integrity (CPI) is proud to work very closely everyday with our law enforcement partners to stop exploitation of vulnerable patients and misuse of taxpayer dollars,” said Deputy Administrator and CPI Director Alec Alexander. “In this case CMS has taken swift administrative action and has suspended payments to 130 distinct providers thereby likely preventing billions of additional dollars in losses. CMS remains committed to protecting the millions of beneficiaries we are honored to serve and to preventing fraud of all sorts in the Medicare and Medicaid programs.”
According to allegations in court documents, some of the defendants obtained patients for the scheme by using an international call center that advertised to Medicare beneficiaries and “up-sold” the beneficiaries to get them to accept numerous “free or low-cost” DME braces, regardless of medical necessity. The international call center allegedly paid illegal kickbacks and bribes to telemedicine companies to obtain DME orders for these Medicare beneficiaries. The telemedicine companies then allegedly paid physicians to write medically unnecessary DME orders. Finally, the international call center sold the DME orders that it obtained from the telemedicine companies to DME companies, which fraudulently billed Medicare. Collectively, the CEOs, COOs, executives, business owners and medical professionals involved in the conspiracy are accused of causing over $1 billion in loss.
The Fraud Section leads the Medicare Fraud Strike Force. Since its inception in March 2007, the Medicare Fraud Strike Force, which maintains 14 strike forces operating in 23 districts, has charged nearly 4,000 defendants who have collectively billed the Medicare program for more than $14 billion. In addition, the HHS Centers for Medicare & Medicaid Services, working in conjunction with the HHS-OIG, are taking steps to increase accountability and decrease the presence of fraudulent providers.
Amongst those charged by Strike Force attorneys include:
In the District of New Jersey, charges were brought against Creaghan Harry, 51, of Highland Beach, Florida; Lester Stockett, 51, of Deefield Beach, Florida; and Elliot Loewenstern, 56, of Boca Raton, Florida; the owner, CEO and VP of marketing, respectively, of purported call centers and telemedicine companies, for their alleged participation in a $454 million illegal healthcare kickback and international money laundering scheme related to the solicitation of illegal kickbacks and bribes in exchange for the referral of DME orders to DME providers. In addition, Joseph DeCoroso, M.D., 62, of Toms River, New Jersey, was charged in a $13 million conspiracy to commit healthcare fraud and separate charges of healthcare fraud for writing medically unnecessary orders for DME, in many instances without ever speaking to the patients, while working for two telemedicine companies. The cases are being prosecuted by Fraud Section Acting Assistant Chief Jacob Foster and Trial Attorney Darren Halverson.
In the Middle District of Florida, charges were brought against Willie McNeal, 42, of Spring Hill, Florida, the owner and CEO of two purported telemedicine companies, for his alleged participation in a $250 million scheme related to the solicitation of illegal kickbacks and bribes in exchange for the referral of DME orders to DME providers. The case is being prosecuted by Fraud Section Acting Assistant Chief Jacob Foster and Trial Attorneys John Michelich, Catherine Wagner and Sara Clingan.
In the Northern District of Texas, charges were brought against Leah Hagen, 48, and Michael Hagen, 51, of Dalworthington Gardens, Texas, owners and operators of two DME companies, for their alleged participation in a $17 million illegal healthcare kickback scheme related to the payment of kickbacks in exchange for the referral of medically unnecessary DME orders. The case is being prosecuted by Fraud Section Trial Attorneys Brynn Schiess and Carlos Lopez.
In the Western District of Texas, Christopher O’Hara, 54, of Kingsbury, Texas, the owner of a purported telemedicine company, was charged in an $40 million scheme related to the alleged solicitation of illegal kickbacks and bribes in exchange for the referral of DME orders to DME providers. The case is being prosecuted by Fraud Section Trial Attorney Kevin Lowell.
In the Eastern District of Pennsylvania, Randy Swackhammer, M.D., 60, of Goldsboro, North Carolina, was charged for an alleged $5 million conspiracy to commit healthcare fraud that involved writing medically unnecessary orders for DME while working for a telemedicine company, in many instances with only a brief telephonic conversation with the patients. The case is being prosecuted by Fraud Section Trial Attorney Adam Yoffie.
In the Central District of California, charges were brought against Darin Flashberg, 41, of Glendora, California, and Najib Jabbour, 47, of Glendora, California, owners of seven DME companies, for their alleged participation in a $34 million scheme related to their payment of kickbacks and bribes in exchange for medically unnecessary DME orders. The case is being prosecuted by Fraud Section Trial Attorney Robyn Pullio.
In addition to the Strike Force prosecutions, other enforcement actions were taken, including the execution of search warrants to support related investigative efforts in seven additional U.S. Attorney’s Offices to include in various investigations conducted by the District of New Jersey, District of South Carolina, Southern District of California, District of Nebraska, Middle District of Florida, Eastern District of Missouri and Western District of Washington.
In the District of South Carolina, charges were brought against Andrew Chmiel, 43, of Mt. Pleasant, South Carolina, owner of over a dozen companies involved in the scheme, for his alleged participation in a $200 million scheme related to the payment of kickbacks and bribes in exchange for medically unnecessary DME orders. The cases are being prosecuted by Assistant U.S. Attorneys Jim May and Will Lewis of the District of South Carolina.
In the District of New Jersey, charges were brought against Neal Williamsky 59, of Marlboro, New Jersey, and Nadia Levit, 39, of Englishtown, New Jersey, owners of approximately 25 DME companies, for their alleged participation in a $150 million scheme related to the payment of kickbacks and bribes in exchange for medically unnecessary DME orders. Albert Davydov, 26, of Rego Park, New York, was also charged for his alleged participation in a $35 million scheme related to the payment of kickbacks and bribes in exchange for medically unnecessary DME orders. The cases are being prosecuted by Assistant U.S. Attorneys Brian Urbano and Stephen Ferketic of the District of New Jersey.
In the Middle District of Florida, search and seizure warrants are being executed at 20 different business locations, including numerous DME companies and a fraudulent telemarketing company. The search and seizures are being executed by over 100 law-enforcement officers from six federal agencies, including HHS-OIG, FBI, IRS-CI, VA-OIG, SSA-OIG, and USPS-OIG. In addition to the 20 search warrants, millions of dollars and other assets tied to the conspiracy are being seized and/or frozen, including through a civil injunction naming 13 defendants as authorized under 18 U.S.C. § 1345.
The cases announced are being prosecuted and investigated by U.S. Attorney’s Offices nationwide, along with MFSF teams from the Criminal Division’s Fraud Section and from the U.S. Attorney’s Offices in the District of New Jersey, District of South Carolina, Southern District of California, District of Nebraska, Middle District of Florida, Eastern District of Missouri and Western District of Washington; and agents from the FBI, HHS-OIG, IRS-CI and other federal law enforcement agencies.
A complaint, information or indictment is merely an allegation, and all defendants are presumed innocent until proven guilty beyond a reasonable doubt in a court of law.
Any doctors or medical professionals who have been involved with alleged fraudulent telemedicine and DME marketing schemes—including Video Doctor USA, AffordADoc, Web Doctors Plus, Integrated Support Plus and First Care MD—should call to report this conduct to the FBI hotline at 1-800-CALL-FBI.
Additional documents related to this announcement will shortly be available here: https://www.justice.gov/opa/documents-and-resources-april-9-2019-press-release-health-care-fraud.
Assistant Attorney General Brian A. Benczkowski of the Justice Department’s Criminal Division, U.S. Attorney Sherri A. Lydon of the District of South Carolina, U.S. Attorney Craig Carpenito of the District of New Jersey, U.S. Attorney Maria Chapa Lopez of the Middle District of Florida, Assistant Director Robert Johnson of the FBI’s Criminal Investigative Division, Deputy Inspector General for Investigations Gary Cantrell of the U.S. Department of Health and Human Services Office of Inspector General (HHS-OIG), Chief Don Fort of the IRS Criminal Investigation (CI) and Deputy Administrator and Director of CPI Alec Alexander of the CMS/CPI made the announcement.
The enforcement actions were led and coordinated by the Health Care Fraud Unit of the Criminal Division’s Fraud Section in conjunction with its Medicare Fraud Strike Force (MFSF), as well as the U.S. Attorney’s Offices for the Districts of South Carolina, New Jersey and the Middle District of Florida. The MFSF is a partnership among the Criminal Division, U.S. Attorney’s Offices, the FBI and HHS-OIG. In addition, IRS-CI and other federal law enforcement agencies participated in the operation.
The charges announced target an alleged scheme involving the payment of illegal kickbacks and bribes by DME companies in exchange for the referral of Medicare beneficiaries by medical professionals working with fraudulent telemedicine companies for back, shoulder, wrist and knee braces that are medically unnecessary. Some of the defendants allegedly controlled an international telemarketing network that lured over hundreds of thousands of elderly and/or disabled patients into a criminal scheme that crossed borders, involving call centers in the Philippines and throughout Latin America. The defendants allegedly paid doctors to prescribe DME either without any patient interaction or with only a brief telephonic conversation with patients they had never met or seen. The proceeds of the fraudulent scheme were allegedly laundered through international shell corporations and used to purchase exotic automobiles, yachts and luxury real estate in the United States and abroad.
“These defendants—who range from corporate executives to medical professionals—allegedly participated in an expansive and sophisticated fraud to exploit telemedicine technology meant for patients otherwise unable to access healthcare,” said Assistant Attorney General Benczkowski. “This Department of Justice will not tolerate medical professionals and executives who look to line their pockets by cheating our healthcare programs. I commend the Criminal Division prosecutors and our partners from U.S. Attorney’s Offices and law enforcement agencies across the country for their unrelenting efforts to stop this alleged fraud before more money was stolen from American taxpayers.”
“Simply put, the law applies equally to all in South Carolina,” said U.S. Attorney Sherri Lydon. “The same spoon that serves indictments on drug dealers, felons in possession of firearms, and corrupt officials will also feed those companies and individuals who engage in Medicare fraud. White collar crime is not victimless. All taxpayers will endure the rising cost of healthcare premiums and out-of-pocket costs as a result of fraud on our Medicare system. I am honored to stand with our partners at the FBI, HHS-OIG, and IRS-CI, who led this outstanding and nationally significant investigation from right here in South Carolina.”
“The indictments we are unsealing charge the defendants with running a complex, multilayered scheme to defraud our Medicare system and avoid detection by government regulators,” said U.S. Attorney Craig Carpenito. “The defendants took advantage of unwitting patients who were simply trying to get relief from their health concerns. Instead, the defendants preyed upon their weakened state and pushed millions of dollars’ worth of unnecessary medical devices, which Medicare paid for, and then set up an elaborate system for laundering their ill-gotten proceeds. We are proud to join our law enforcement partners in New Jersey and around the country to put a stop to this unscrupulous criminal activity.”
“Protecting the integrity of America’s healthcare programs is necessary to ensure that our citizens receive the care they have paid for and deserve,” said U.S. Attorney Chapa Lopez. “The mammoth coordination and cooperation demonstrated among the various offices, districts, and agencies involved in this case leaves no doubt. We will leverage the full weight of our resources to combat fraud and abuse, wherever it is found.”
“One of the largest healthcare fraud schemes in U.S. history came to an end thanks to close collaboration and coordination between the FBI and partners including HHS-OIG and IRS-CI,” said FBI Assistant Director Robert Johnson. “Healthcare fraud causes billions of dollars in losses, it deprives real patients of the critical healthcare services they need, and it can endanger the lives of real patients so individuals like those arrested can profit from their criminal activity. Through the coordinated national effort, we put an end to this egregious and costly healthcare fraud scheme, and the public can rest assured the FBI will continue to make healthcare fraud investigations a top priority.”
“Our law enforcement officers are focused on preventing and uprooting healthcare fraud schemes like those alleged,” said Deputy Inspector General for Investigations Gary Cantrell. “These schemes divert money from taxpayer-funded federal healthcare programs into the hands of criminals. Working closely with our law enforcement partners, our agency will continue to investigate and disrupt attempts to undermine Medicare and target beneficiaries.”
“The breadth of this nationwide conspiracy should be frightening to all who rely on some form of healthcare,” said IRS-CI Chief Don Fort. “The conspiracy described in this indictment was not perpetrated by one individual. Rather, it details broad corruption, massive amounts of greed, and systemic flaws in our healthcare system that were exploited by the defendants. We all suffer when schemes like this go undiscovered and I’m proud of the work our agents did in working with our partners to uncover this complex scheme.”
“The Centers for Medicare & Medicaid Services (CMS) Center for Program Integrity (CPI) is proud to work very closely everyday with our law enforcement partners to stop exploitation of vulnerable patients and misuse of taxpayer dollars,” said Deputy Administrator and CPI Director Alec Alexander. “In this case CMS has taken swift administrative action and has suspended payments to 130 distinct providers thereby likely preventing billions of additional dollars in losses. CMS remains committed to protecting the millions of beneficiaries we are honored to serve and to preventing fraud of all sorts in the Medicare and Medicaid programs.”
According to allegations in court documents, some of the defendants obtained patients for the scheme by using an international call center that advertised to Medicare beneficiaries and “up-sold” the beneficiaries to get them to accept numerous “free or low-cost” DME braces, regardless of medical necessity. The international call center allegedly paid illegal kickbacks and bribes to telemedicine companies to obtain DME orders for these Medicare beneficiaries. The telemedicine companies then allegedly paid physicians to write medically unnecessary DME orders. Finally, the international call center sold the DME orders that it obtained from the telemedicine companies to DME companies, which fraudulently billed Medicare. Collectively, the CEOs, COOs, executives, business owners and medical professionals involved in the conspiracy are accused of causing over $1 billion in loss.
The Fraud Section leads the Medicare Fraud Strike Force. Since its inception in March 2007, the Medicare Fraud Strike Force, which maintains 14 strike forces operating in 23 districts, has charged nearly 4,000 defendants who have collectively billed the Medicare program for more than $14 billion. In addition, the HHS Centers for Medicare & Medicaid Services, working in conjunction with the HHS-OIG, are taking steps to increase accountability and decrease the presence of fraudulent providers.
Amongst those charged by Strike Force attorneys include:
In the District of New Jersey, charges were brought against Creaghan Harry, 51, of Highland Beach, Florida; Lester Stockett, 51, of Deefield Beach, Florida; and Elliot Loewenstern, 56, of Boca Raton, Florida; the owner, CEO and VP of marketing, respectively, of purported call centers and telemedicine companies, for their alleged participation in a $454 million illegal healthcare kickback and international money laundering scheme related to the solicitation of illegal kickbacks and bribes in exchange for the referral of DME orders to DME providers. In addition, Joseph DeCoroso, M.D., 62, of Toms River, New Jersey, was charged in a $13 million conspiracy to commit healthcare fraud and separate charges of healthcare fraud for writing medically unnecessary orders for DME, in many instances without ever speaking to the patients, while working for two telemedicine companies. The cases are being prosecuted by Fraud Section Acting Assistant Chief Jacob Foster and Trial Attorney Darren Halverson.
In the Middle District of Florida, charges were brought against Willie McNeal, 42, of Spring Hill, Florida, the owner and CEO of two purported telemedicine companies, for his alleged participation in a $250 million scheme related to the solicitation of illegal kickbacks and bribes in exchange for the referral of DME orders to DME providers. The case is being prosecuted by Fraud Section Acting Assistant Chief Jacob Foster and Trial Attorneys John Michelich, Catherine Wagner and Sara Clingan.
In the Northern District of Texas, charges were brought against Leah Hagen, 48, and Michael Hagen, 51, of Dalworthington Gardens, Texas, owners and operators of two DME companies, for their alleged participation in a $17 million illegal healthcare kickback scheme related to the payment of kickbacks in exchange for the referral of medically unnecessary DME orders. The case is being prosecuted by Fraud Section Trial Attorneys Brynn Schiess and Carlos Lopez.
In the Western District of Texas, Christopher O’Hara, 54, of Kingsbury, Texas, the owner of a purported telemedicine company, was charged in an $40 million scheme related to the alleged solicitation of illegal kickbacks and bribes in exchange for the referral of DME orders to DME providers. The case is being prosecuted by Fraud Section Trial Attorney Kevin Lowell.
In the Eastern District of Pennsylvania, Randy Swackhammer, M.D., 60, of Goldsboro, North Carolina, was charged for an alleged $5 million conspiracy to commit healthcare fraud that involved writing medically unnecessary orders for DME while working for a telemedicine company, in many instances with only a brief telephonic conversation with the patients. The case is being prosecuted by Fraud Section Trial Attorney Adam Yoffie.
In the Central District of California, charges were brought against Darin Flashberg, 41, of Glendora, California, and Najib Jabbour, 47, of Glendora, California, owners of seven DME companies, for their alleged participation in a $34 million scheme related to their payment of kickbacks and bribes in exchange for medically unnecessary DME orders. The case is being prosecuted by Fraud Section Trial Attorney Robyn Pullio.
In addition to the Strike Force prosecutions, other enforcement actions were taken, including the execution of search warrants to support related investigative efforts in seven additional U.S. Attorney’s Offices to include in various investigations conducted by the District of New Jersey, District of South Carolina, Southern District of California, District of Nebraska, Middle District of Florida, Eastern District of Missouri and Western District of Washington.
In the District of South Carolina, charges were brought against Andrew Chmiel, 43, of Mt. Pleasant, South Carolina, owner of over a dozen companies involved in the scheme, for his alleged participation in a $200 million scheme related to the payment of kickbacks and bribes in exchange for medically unnecessary DME orders. The cases are being prosecuted by Assistant U.S. Attorneys Jim May and Will Lewis of the District of South Carolina.
In the District of New Jersey, charges were brought against Neal Williamsky 59, of Marlboro, New Jersey, and Nadia Levit, 39, of Englishtown, New Jersey, owners of approximately 25 DME companies, for their alleged participation in a $150 million scheme related to the payment of kickbacks and bribes in exchange for medically unnecessary DME orders. Albert Davydov, 26, of Rego Park, New York, was also charged for his alleged participation in a $35 million scheme related to the payment of kickbacks and bribes in exchange for medically unnecessary DME orders. The cases are being prosecuted by Assistant U.S. Attorneys Brian Urbano and Stephen Ferketic of the District of New Jersey.
In the Middle District of Florida, search and seizure warrants are being executed at 20 different business locations, including numerous DME companies and a fraudulent telemarketing company. The search and seizures are being executed by over 100 law-enforcement officers from six federal agencies, including HHS-OIG, FBI, IRS-CI, VA-OIG, SSA-OIG, and USPS-OIG. In addition to the 20 search warrants, millions of dollars and other assets tied to the conspiracy are being seized and/or frozen, including through a civil injunction naming 13 defendants as authorized under 18 U.S.C. § 1345.
The cases announced are being prosecuted and investigated by U.S. Attorney’s Offices nationwide, along with MFSF teams from the Criminal Division’s Fraud Section and from the U.S. Attorney’s Offices in the District of New Jersey, District of South Carolina, Southern District of California, District of Nebraska, Middle District of Florida, Eastern District of Missouri and Western District of Washington; and agents from the FBI, HHS-OIG, IRS-CI and other federal law enforcement agencies.
A complaint, information or indictment is merely an allegation, and all defendants are presumed innocent until proven guilty beyond a reasonable doubt in a court of law.
Any doctors or medical professionals who have been involved with alleged fraudulent telemedicine and DME marketing schemes—including Video Doctor USA, AffordADoc, Web Doctors Plus, Integrated Support Plus and First Care MD—should call to report this conduct to the FBI hotline at 1-800-CALL-FBI.
Additional documents related to this announcement will shortly be available here: https://www.justice.gov/opa/documents-and-resources-april-9-2019-press-release-health-care-fraud.