Business Wire02.11.19
Current Health, an artificial intelligence (AI)-powered wearable, announced it has received Class II clearance from the U.S. Food and Drug Administration (FDA) for hospital care. Current is a wireless device that continuously and automatically monitors patients to help better determine health trajectory and allows clinicians to intervene earlier.
Faced with an aging population and strained healthcare systems worldwide, U.S. and U.K. healthcare providers are deploying Current to change their patient delivery models from reactive to proactive care to produce better patient outcomes. Current’s approach will help health organizations reduce unnecessary hospital readmissions for patients whose conditions deteriorate after treatment—an expensive and cumbersome clinical burden that costs U.S. hospitals more than $40 billion annually.
Mount Sinai Brooklyn is working with Current to detect patient deterioration earlier and improve healthcare outcomes, resulting in the safest and highest quality care for patients. Dr. Scott Lorin, president of Mount Sinai Brooklyn, said: “The Mount Sinai Health System works with innovative and leading-edge companies like Current to support our commitment to providing world-class patient care. Current’s continuous and proactive monitoring platform has the potential to alert us to patient deterioration faster and give our team data insights they can act on earlier.”
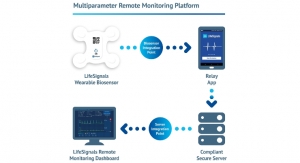
Built using the world’s largest real-time physiological data set, Current is the most accurate, all-in-one wireless wearable currently approved for use in the EU and U.S. The company’s proprietary algorithms continuously analyze data, along with relevant contextual patient information, to offer actionable and proactive insights into the wearer’s health. It seamlessly integrates with third-party devices to capture additional metrics, building patient-specific digital therapeutics and recommendations.
Current is actively in use with U.K. healthcare providers in a post-acute setting, including Dartford and Gravesham NHS Trust, which serves a local population of 500,000 people. The Dartford and Gravesham NHS Trust Hospital at Home team uses Current to remotely monitor patients after discharge. As a result, clinicians and staff reprioritized home visits based on criticality, resulting in a 22 percent reduction in home visits and fewer hospital readmissions and emergency department visits, which freed up skilled nursing time and helped patients feel safe and secure.
“At Current, we’re a small team of individuals committed to changing the world through proactive healthcare,” said Christopher McCann, CEO of Current. “Our team worked hard to get here, and it’s just the first step toward monitoring the health of every human being to identify sickness earlier with the goal of saving lives. Today, we’re in the hospital, tomorrow the home, and in the near future, we’ll be everywhere. We are just getting started.”
Current is demonstrating its FDA-cleared solution at the HIMSS19 trade show in Orlando from February 11 to 14 at Booth 5359-05. Additionally, CEO Christopher McCann is presenting a talk titled, “Deriving actionable insights from RPM and telehealth” on Wednesday, February 13, at 12:45 p.m. ET at the Healthcare of the Future Pavilion #5359.
Founded in 2014, Current is headquartered in Edinburgh, Scotland with an office in New York. The team is led by co-founders Christopher McCann and Stewart Whiting and is comprised of Ph.D.s, clinical researchers, healthcare professionals, data scientists, and engineering experts.
Faced with an aging population and strained healthcare systems worldwide, U.S. and U.K. healthcare providers are deploying Current to change their patient delivery models from reactive to proactive care to produce better patient outcomes. Current’s approach will help health organizations reduce unnecessary hospital readmissions for patients whose conditions deteriorate after treatment—an expensive and cumbersome clinical burden that costs U.S. hospitals more than $40 billion annually.
Mount Sinai Brooklyn is working with Current to detect patient deterioration earlier and improve healthcare outcomes, resulting in the safest and highest quality care for patients. Dr. Scott Lorin, president of Mount Sinai Brooklyn, said: “The Mount Sinai Health System works with innovative and leading-edge companies like Current to support our commitment to providing world-class patient care. Current’s continuous and proactive monitoring platform has the potential to alert us to patient deterioration faster and give our team data insights they can act on earlier.”
Built using the world’s largest real-time physiological data set, Current is the most accurate, all-in-one wireless wearable currently approved for use in the EU and U.S. The company’s proprietary algorithms continuously analyze data, along with relevant contextual patient information, to offer actionable and proactive insights into the wearer’s health. It seamlessly integrates with third-party devices to capture additional metrics, building patient-specific digital therapeutics and recommendations.
Current is actively in use with U.K. healthcare providers in a post-acute setting, including Dartford and Gravesham NHS Trust, which serves a local population of 500,000 people. The Dartford and Gravesham NHS Trust Hospital at Home team uses Current to remotely monitor patients after discharge. As a result, clinicians and staff reprioritized home visits based on criticality, resulting in a 22 percent reduction in home visits and fewer hospital readmissions and emergency department visits, which freed up skilled nursing time and helped patients feel safe and secure.
“At Current, we’re a small team of individuals committed to changing the world through proactive healthcare,” said Christopher McCann, CEO of Current. “Our team worked hard to get here, and it’s just the first step toward monitoring the health of every human being to identify sickness earlier with the goal of saving lives. Today, we’re in the hospital, tomorrow the home, and in the near future, we’ll be everywhere. We are just getting started.”
Current is demonstrating its FDA-cleared solution at the HIMSS19 trade show in Orlando from February 11 to 14 at Booth 5359-05. Additionally, CEO Christopher McCann is presenting a talk titled, “Deriving actionable insights from RPM and telehealth” on Wednesday, February 13, at 12:45 p.m. ET at the Healthcare of the Future Pavilion #5359.
Founded in 2014, Current is headquartered in Edinburgh, Scotland with an office in New York. The team is led by co-founders Christopher McCann and Stewart Whiting and is comprised of Ph.D.s, clinical researchers, healthcare professionals, data scientists, and engineering experts.