Business Wire01.29.19
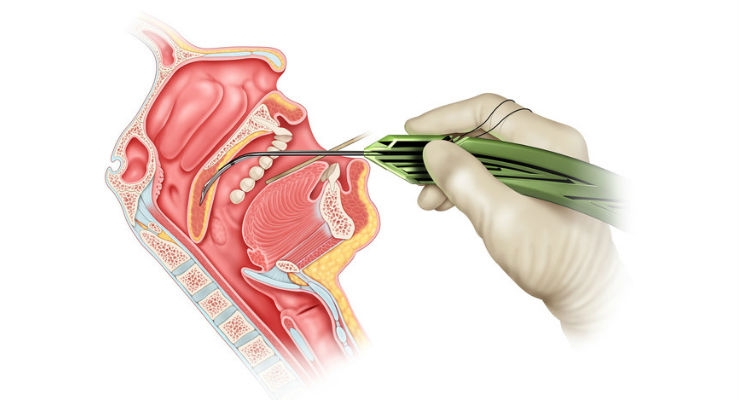
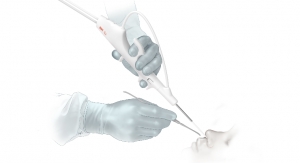
Cook Medical and Zelegent Inc., announced an FDA clearance for the ElevoKit Snoring Intervention Device. The specialized tool is designed to enhance patient treatment options for snoring. Elevo’s delivery system allows placement of specially-shaped sutures that provide lift to the soft palate and then are naturally dissolved by the body. It is indicated for symptomatic, habitual, and social snoring due to palatal flutter.
Elevo is used in Elevoplasty, a procedure that provides lift and stiffening to the soft palate tissue in the mouth. Elevoplasty can be performed under local anesthesia in an office setting without the need for surgery.
“Elevoplasty is like a mini-facelift for the soft palate,” said Alexander K. Arrow, M.D., chief executive officer of Zelegent. “We are honored to have the Elevo product adopted into a minimally invasive and innovative Cook product portfolio whose reputation dates back to Cook’s early work supporting pioneering physicians.”
Zelegent recently completed a multi-center clinical trial designed to evaluate the safety and efficacy of snoring intervention with Elevoplasty in an office-based setting. The S.I.L.E.N.C.E. clinical study included both academic institutions and private practitioners, including several thought leaders of sleep disorder treatments and international lecturers on sleep surgery in the field of otolaryngology.
“Approximately 20 percent of adults in the developed world consistently snore at night, at volumes high enough to disturb their sleeping partners,” said Thomas Cherry, OHNS global business leader. “This can be damaging to one’s quality of sleep, as well as their relationships. We’re very excited to provide a minimally invasive, office-based procedure to treat patients with this condition.”
Cook will begin training physicians on the Elevo Kit later this year.
Elevo is used in Elevoplasty, a procedure that provides lift and stiffening to the soft palate tissue in the mouth. Elevoplasty can be performed under local anesthesia in an office setting without the need for surgery.
“Elevoplasty is like a mini-facelift for the soft palate,” said Alexander K. Arrow, M.D., chief executive officer of Zelegent. “We are honored to have the Elevo product adopted into a minimally invasive and innovative Cook product portfolio whose reputation dates back to Cook’s early work supporting pioneering physicians.”
Zelegent recently completed a multi-center clinical trial designed to evaluate the safety and efficacy of snoring intervention with Elevoplasty in an office-based setting. The S.I.L.E.N.C.E. clinical study included both academic institutions and private practitioners, including several thought leaders of sleep disorder treatments and international lecturers on sleep surgery in the field of otolaryngology.
“Approximately 20 percent of adults in the developed world consistently snore at night, at volumes high enough to disturb their sleeping partners,” said Thomas Cherry, OHNS global business leader. “This can be damaging to one’s quality of sleep, as well as their relationships. We’re very excited to provide a minimally invasive, office-based procedure to treat patients with this condition.”
Cook will begin training physicians on the Elevo Kit later this year.