Business Wire10.08.18
Vascular Dynamics Inc. (VDI), a privately held medical device company focused on minimally invasive device-based solutions for cardiovascular conditions, has enrolled the first patients in a U.S. Food and Drug Administration (FDA)-approved best-in-class pivotal clinical trial. The CALM-2 study is designed to establish safety and efficacy of the novel endovascular baroreceptor amplification (EVBA) procedure using the unique MobiusHD device as a treatment for drug-resistant hypertension.
Over 100 million people in the United States have hypertension, which is recognized as the most important single risk factor for cardiovascular morbidity and death. Existing pharmacological interventions are effective for some, but more than half of those treated with antihypertensive medications continue to have uncontrolled high blood pressure.
“I am optimistic that device-based treatments, such as EVBA with the MobiusHD implant, may be able to provide effective solutions for patients who have not benefited from drug-based treatments,” said professor Bryan Williams, co-principal investigator of the trial and chair of Medicine at University College London, director of Research at UCL Hospitals, and chairman-elect of the European Council on Hypertension of the European Society of Cardiology.
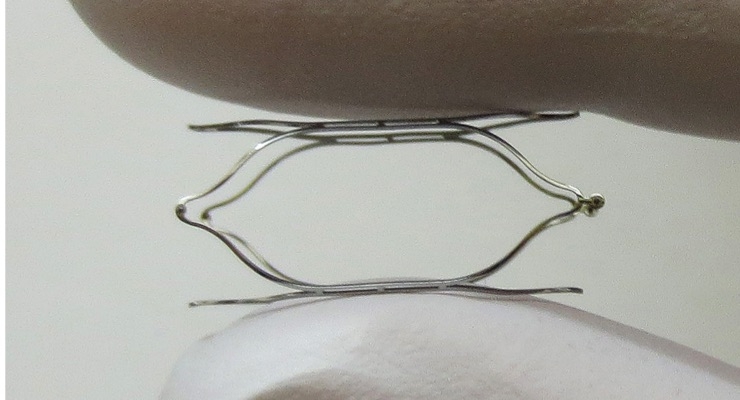
Baroreceptors are specialized nerves located in the carotid artery, which detect changes in stretch of the arterial wall and signal the brain to control blood pressure. The baroreceptor mechanism, or baroreflex, is an essential component for the body’s natural regulation of blood pressure. VDI is developing the first minimally invasive technology designed to use this natural blood pressure control system to address uncontrolled hypertension. The MobiusHD is a flexible self-expanding device that reshapes the carotid sinus following endovascular implantation. It is intended to amplify the baroreflex while maintaining pulsatility.
Results published in The Lancet from an open-label, proof-of-concept trial (CALM-FIM) using VDI’s EVBA approach to treat patients with resistant hypertension were positive, showing significant reductions in blood pressure through six months, greater than the 24-hour ambulatory blood pressure reductions reported to date for alternative devices used to treat hypertension.1 These results led to the design and development of a rigorous pivotal clinical trial intended to generate conclusive evidence confirming the safety and efficacy of this unique device for resistant hypertension patients.
The CALM-2 (Controlling And Lowering blood pressure with MobiusHD) pivotal trial is a prospective, randomized, sham-controlled, double-blinded study targeting patients with drug-resistant hypertension. Lessons learned from numerous clinical trials of first-generation device-based approaches to hypertension were incorporated into the CALM-2 study protocol to provide a best-in-class clinical trial design. The CALM-2 trial is targeting enrollment of up to 300 patients at leading institutions across the United States and Europe. The first patients were enrolled in July 2018 at the Center for Clinical Research at Southern Illinois University Medicine and The Lindner Research Center at The Christ Hospital Health Network.
“Enrolling the first patients in the CALM-2 trial is an important step toward offering a new treatment option for this large patient population with resistant hypertension and the associated health risks,” stated Gregg Stone, M.D., co-principal investigator of the CALM-2 trial and director of Cardiovascular Research and Education for NewYork-Presbyterian Hospital/Columbia University Medical Center and co-director of Medical Research and Education at the Cardiovascular Research Foundation.
VDI’s recently appointed president and CEO Ed Roschak said, “We are putting the right team and processes in place to successfully complete this landmark trial and to develop compelling clinical evidence to support future FDA approval.”
Vascular Dynamics develops minimally invasive platform technologies for treating patients at risk of life-threatening cardiovascular events and underserved by conventional treatments. Focused initially on uncontrolled hypertension, VDI was approved to participate in the FDA's Expedited Access Pathway (EAP) program. EVBA treatment with the MobiusHD implant system has received a CE Mark for the treatment of hypertension in the European Union. The MobiusHD system is not commercially available in the United States.
The MobiusHD device is limited in the United States to investigational use only.
References
1. Spiering, W, Williams, B, Van der Heyden, J…, Endovascular baroreflex amplification for the resistant hypertension: a safety and proof-of-principle clinical study, The Lancet 2017.
Over 100 million people in the United States have hypertension, which is recognized as the most important single risk factor for cardiovascular morbidity and death. Existing pharmacological interventions are effective for some, but more than half of those treated with antihypertensive medications continue to have uncontrolled high blood pressure.
“I am optimistic that device-based treatments, such as EVBA with the MobiusHD implant, may be able to provide effective solutions for patients who have not benefited from drug-based treatments,” said professor Bryan Williams, co-principal investigator of the trial and chair of Medicine at University College London, director of Research at UCL Hospitals, and chairman-elect of the European Council on Hypertension of the European Society of Cardiology.
Baroreceptors are specialized nerves located in the carotid artery, which detect changes in stretch of the arterial wall and signal the brain to control blood pressure. The baroreceptor mechanism, or baroreflex, is an essential component for the body’s natural regulation of blood pressure. VDI is developing the first minimally invasive technology designed to use this natural blood pressure control system to address uncontrolled hypertension. The MobiusHD is a flexible self-expanding device that reshapes the carotid sinus following endovascular implantation. It is intended to amplify the baroreflex while maintaining pulsatility.
Results published in The Lancet from an open-label, proof-of-concept trial (CALM-FIM) using VDI’s EVBA approach to treat patients with resistant hypertension were positive, showing significant reductions in blood pressure through six months, greater than the 24-hour ambulatory blood pressure reductions reported to date for alternative devices used to treat hypertension.1 These results led to the design and development of a rigorous pivotal clinical trial intended to generate conclusive evidence confirming the safety and efficacy of this unique device for resistant hypertension patients.
The CALM-2 (Controlling And Lowering blood pressure with MobiusHD) pivotal trial is a prospective, randomized, sham-controlled, double-blinded study targeting patients with drug-resistant hypertension. Lessons learned from numerous clinical trials of first-generation device-based approaches to hypertension were incorporated into the CALM-2 study protocol to provide a best-in-class clinical trial design. The CALM-2 trial is targeting enrollment of up to 300 patients at leading institutions across the United States and Europe. The first patients were enrolled in July 2018 at the Center for Clinical Research at Southern Illinois University Medicine and The Lindner Research Center at The Christ Hospital Health Network.
“Enrolling the first patients in the CALM-2 trial is an important step toward offering a new treatment option for this large patient population with resistant hypertension and the associated health risks,” stated Gregg Stone, M.D., co-principal investigator of the CALM-2 trial and director of Cardiovascular Research and Education for NewYork-Presbyterian Hospital/Columbia University Medical Center and co-director of Medical Research and Education at the Cardiovascular Research Foundation.
VDI’s recently appointed president and CEO Ed Roschak said, “We are putting the right team and processes in place to successfully complete this landmark trial and to develop compelling clinical evidence to support future FDA approval.”
Vascular Dynamics develops minimally invasive platform technologies for treating patients at risk of life-threatening cardiovascular events and underserved by conventional treatments. Focused initially on uncontrolled hypertension, VDI was approved to participate in the FDA's Expedited Access Pathway (EAP) program. EVBA treatment with the MobiusHD implant system has received a CE Mark for the treatment of hypertension in the European Union. The MobiusHD system is not commercially available in the United States.
The MobiusHD device is limited in the United States to investigational use only.
References
1. Spiering, W, Williams, B, Van der Heyden, J…, Endovascular baroreflex amplification for the resistant hypertension: a safety and proof-of-principle clinical study, The Lancet 2017.