Abbott Laboratories09.05.18
Abbott announced a new national coverage determination for the company's dorsal root ganglion (DRG) neurostimulation pain therapy through Aetna, a leading health benefits company in the United States. With this coverage decision, Aetna will provide more than 22 million medical plan members with access to Abbott's DRG therapy for people with chronic pain. Aetna already covers Abbott's spinal cord stimulation therapies. Favorable coverage decisions for neurostimulation therapies by private payers such as Aetna support the goals of the U.S. Food and Drug Administration (FDA), Centers for Medicare & Medicaid (CMS) and other government bodies to improve access to non-opioid alternatives for pain management.
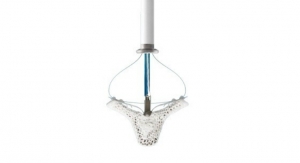
Abbott's DRG therapy is a form of neurostimulation that specifically targets the dorsal root ganglion, a structure along the spinal column which is densely populated with nerves that transmit sensory information to the central nervous system. By blocking or disrupting pain signals transmitted via the DRG, the therapy helps people living with neuropathic pain conditions, some of the most prevalent and under-treated forms of chronic pain in America.
Historically, these conditions have been challenging for physicians to treat because the pain stems from damage to tissue and nerves or results from a disruption in how the peripheral and central nervous systems process or transmit pain signals. Worse, neuropathic pain conditions are often characterized by intense shooting pain or a burning sensation and in their search for pain relief, many people try medication, opioids, and surgery without success.
Abbott's Proclaim DRG system has been shown in more than a dozen clinical studies to provide superior pain relief to patients with neuropathic conditions currently underserved by other forms of chronic pain therapy. Examples of these conditions may include chronic pain following hernia repair, total joint replacements or amputation. Patients are also able to try DRG stimulation with a non-invasive weeklong therapy trial, or temporary evaluation, before committing to an implant procedure.
"Since its launch in the U.S., Abbott's DRG stimulation has had a profound impact for people living with complex pain conditions. One of the most challenging stories we hear is when patients undergo a therapy trial, find pain relief and then learn their insurance won't cover their therapy," said Keith Boettiger, vice president of Abbott's neuromodulation business. "While Medicare already covers our DRG system, it's encouraging to see private payers like Aetna review the clinical data and outcomes, then choose to provide access to DRG stimulation for their members. These decisions build on the momentum of broader national coverage decisions by private insurers and support the FDA's goals to find new ways to combat the opioid epidemic with alternatives such as Abbott's neurostimulation therapies."
Abbott's DRG therapy has an extensive history of rigorous scientific research. More than a dozen clinical studies involving more than 500 patients have been published in peer-reviewed journals. The ACCURATE IDE study of Abbott's DRG therapy was the largest clinical trial to date evaluating patients with complex regional pain syndrome (CRPS) or causalgia. The ACCURATE study, which was used to gain FDA approval, showed that DRG therapy provided significant pain relief to 8 out of 10 people at 12 months. It also showed greater treatment success when compared to patients receiving traditional tonic spinal cord stimulation.
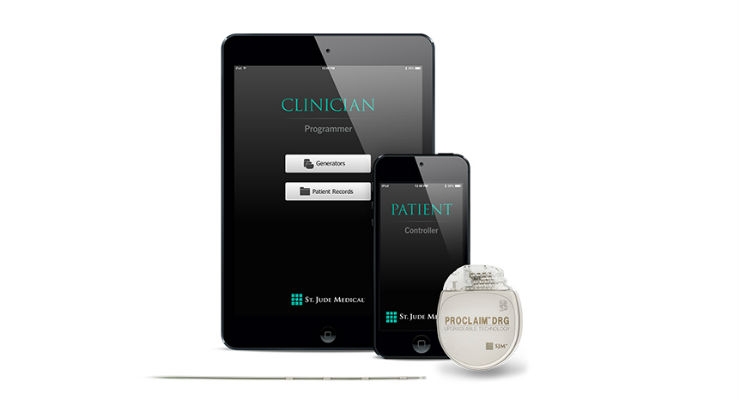
Abbott's Proclaim DRG system is magnetic resonance (MR) labeled for the head and neck, low maintenance and recharge free, meaning patients do not need to be reminded of their condition and pause from their daily activities to charge their battery as with other systems. Using Bluetooth wireless connectivity, patients can feel empowered by controlling their stimulation levels through a familiar Apple iPod touch device. DRG stimulation therapy is available on the Proclaim DRG Neurostimulation System.
Abbott's DRG therapy is a form of neurostimulation that specifically targets the dorsal root ganglion, a structure along the spinal column which is densely populated with nerves that transmit sensory information to the central nervous system. By blocking or disrupting pain signals transmitted via the DRG, the therapy helps people living with neuropathic pain conditions, some of the most prevalent and under-treated forms of chronic pain in America.
Historically, these conditions have been challenging for physicians to treat because the pain stems from damage to tissue and nerves or results from a disruption in how the peripheral and central nervous systems process or transmit pain signals. Worse, neuropathic pain conditions are often characterized by intense shooting pain or a burning sensation and in their search for pain relief, many people try medication, opioids, and surgery without success.
Abbott's Proclaim DRG system has been shown in more than a dozen clinical studies to provide superior pain relief to patients with neuropathic conditions currently underserved by other forms of chronic pain therapy. Examples of these conditions may include chronic pain following hernia repair, total joint replacements or amputation. Patients are also able to try DRG stimulation with a non-invasive weeklong therapy trial, or temporary evaluation, before committing to an implant procedure.
"Since its launch in the U.S., Abbott's DRG stimulation has had a profound impact for people living with complex pain conditions. One of the most challenging stories we hear is when patients undergo a therapy trial, find pain relief and then learn their insurance won't cover their therapy," said Keith Boettiger, vice president of Abbott's neuromodulation business. "While Medicare already covers our DRG system, it's encouraging to see private payers like Aetna review the clinical data and outcomes, then choose to provide access to DRG stimulation for their members. These decisions build on the momentum of broader national coverage decisions by private insurers and support the FDA's goals to find new ways to combat the opioid epidemic with alternatives such as Abbott's neurostimulation therapies."
Abbott's DRG therapy has an extensive history of rigorous scientific research. More than a dozen clinical studies involving more than 500 patients have been published in peer-reviewed journals. The ACCURATE IDE study of Abbott's DRG therapy was the largest clinical trial to date evaluating patients with complex regional pain syndrome (CRPS) or causalgia. The ACCURATE study, which was used to gain FDA approval, showed that DRG therapy provided significant pain relief to 8 out of 10 people at 12 months. It also showed greater treatment success when compared to patients receiving traditional tonic spinal cord stimulation.
Abbott's Proclaim DRG system is magnetic resonance (MR) labeled for the head and neck, low maintenance and recharge free, meaning patients do not need to be reminded of their condition and pause from their daily activities to charge their battery as with other systems. Using Bluetooth wireless connectivity, patients can feel empowered by controlling their stimulation levels through a familiar Apple iPod touch device. DRG stimulation therapy is available on the Proclaim DRG Neurostimulation System.