Abbott Laboratories07.24.18
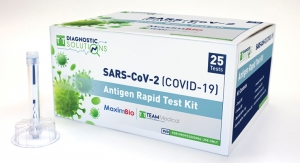
Today, Abbott is proud to introduce the m-PIMA HIV-1/2 VL, the first viral load point-of-care test designed to provide healthcare professionals, especially in remote and underserved communities, with a fast, accurate and easy-to-use test to manage HIV.
By providing viral load test results in less than 70 minutes, this life-changing technology allows patients to get tested and treated in the same visit and then get back to leading their best life.
In recent decades, the global health community has made great strides towards ending the global AIDS epidemic by working to ensure that all people living with HIV know their status, receive antiretroviral therapy (ART) and, through effective disease management, reach an undetectable viral load (VL).1 Moreover, UNAIDS has established a set of collaborative goals to ensure all HIV patients receive the optimal level of care, no matter where they live.
"The integration of point-of-care viral load solutions in healthcare networks will be instrumental in achieving UNAIDS’ 90-90-90 goals," said Professor Matilu Mwau, Director at the Center for Infectious and Parasitic Diseases Control Research, Kenya Medical Research Institute (KEMRI) in Busia, Kenya. "Viral load testing is critical for the monitoring of individual treatment response, the effective use of costly antiretroviral medications, and to track the emergence of resistance in people who are HIV-positive."1
"Abbott is committed to delivering HIV diagnostic solutions that support the UNAIDS agenda and enable life-saving decisions that have a profound impact on people and society," said Damian Halloran, vice president, Infectious Disease-Emerging Markets, Rapid Diagnostics, Abbott. "The m-PIMA HIV-1/2 VL is an example of how Abbott is focused on delivering tools that empower healthcare providers and their patients where and when they need it most."
To provide the most effective HIV treatment and care, the World Health Organization (WHO) recommends that everyone receiving ART undergoes a viral load test at 6 months and 12 months, and annually thereafter, if the individual is stable on ART. Viral load testing is the gold standard for monitoring ART treatment failure.2
Unfortunately, very few1 people in resource-limited settings, such as select countries in sub-Saharan Africa, Asia and Latin America, receive this necessary level of care due to limited laboratory infrastructure, shortage of skilled clinical and laboratory staff, weak specimen transport systems, inefficient systems for providing results and patients lost to follow-up. These are the exact challenges the m-PIMA HIV-1/2 VL has been designed to address. The m-PIMA platform is portable and robust, allowing the provision of healthcare in the most challenging of settings. Data Point connectivity supports decentralized program management so ministers of health are able to monitor and raise the standard of care nationwide.
The m-PIMA HIV-1/2 VL is a quantitative nucleic acid amplification test for viral load measurement of HIV type 1 groups M/N and O, and HIV-2 in plasma samples. The test is easy to use, deployable at the point of care and is designed to measure viral load in under 70 minutes, while the patient is still present. The impact is that every patient that needs a VL test can get one in the same visit, allowing more immediate treatment decisions, and consequently helping to reduce mental, emotional and physical burdens.
The m-PIMA HIV-1/2 VL joins Abbott’s comprehensive portfolio of diagnostic solutions that span the entire HIV cascade of care, serving more than 120 countries globally and all 55 countries on the African continent.
Abbott has a long and deep heritage in HIV diagnostics, having introduced the world’s first HIV test in 1985. With a leading set of solutions for screening, monitoring, management and connectivity—from the lab to the point of care, Abbott is providing critical tools to healthcare providers, especially in resource-limited settings.
The m-PIMA HIV-1/2 VL test is now commercially available in select countries. Data has been submitted for CE-IVD marking and WHO prequalification. This product is not available in the United States.
References
1 UNAIDS (2014) 90-90-90 An ambitious target to help end the AIDS epidemic. Available on http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf
2 Roberts, T., Cohn, J., Bonner, K., & Hargreaves, S. (2016). Scale-up of Routine Viral Load Testing in Resource-Poor Settings: Current and Future Implementation Challenges. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 62(8), 1043-1048. http://doi.org/10.1093/cid/ciw001
By providing viral load test results in less than 70 minutes, this life-changing technology allows patients to get tested and treated in the same visit and then get back to leading their best life.
In recent decades, the global health community has made great strides towards ending the global AIDS epidemic by working to ensure that all people living with HIV know their status, receive antiretroviral therapy (ART) and, through effective disease management, reach an undetectable viral load (VL).1 Moreover, UNAIDS has established a set of collaborative goals to ensure all HIV patients receive the optimal level of care, no matter where they live.
"The integration of point-of-care viral load solutions in healthcare networks will be instrumental in achieving UNAIDS’ 90-90-90 goals," said Professor Matilu Mwau, Director at the Center for Infectious and Parasitic Diseases Control Research, Kenya Medical Research Institute (KEMRI) in Busia, Kenya. "Viral load testing is critical for the monitoring of individual treatment response, the effective use of costly antiretroviral medications, and to track the emergence of resistance in people who are HIV-positive."1
"Abbott is committed to delivering HIV diagnostic solutions that support the UNAIDS agenda and enable life-saving decisions that have a profound impact on people and society," said Damian Halloran, vice president, Infectious Disease-Emerging Markets, Rapid Diagnostics, Abbott. "The m-PIMA HIV-1/2 VL is an example of how Abbott is focused on delivering tools that empower healthcare providers and their patients where and when they need it most."
To provide the most effective HIV treatment and care, the World Health Organization (WHO) recommends that everyone receiving ART undergoes a viral load test at 6 months and 12 months, and annually thereafter, if the individual is stable on ART. Viral load testing is the gold standard for monitoring ART treatment failure.2
Unfortunately, very few1 people in resource-limited settings, such as select countries in sub-Saharan Africa, Asia and Latin America, receive this necessary level of care due to limited laboratory infrastructure, shortage of skilled clinical and laboratory staff, weak specimen transport systems, inefficient systems for providing results and patients lost to follow-up. These are the exact challenges the m-PIMA HIV-1/2 VL has been designed to address. The m-PIMA platform is portable and robust, allowing the provision of healthcare in the most challenging of settings. Data Point connectivity supports decentralized program management so ministers of health are able to monitor and raise the standard of care nationwide.
The m-PIMA HIV-1/2 VL is a quantitative nucleic acid amplification test for viral load measurement of HIV type 1 groups M/N and O, and HIV-2 in plasma samples. The test is easy to use, deployable at the point of care and is designed to measure viral load in under 70 minutes, while the patient is still present. The impact is that every patient that needs a VL test can get one in the same visit, allowing more immediate treatment decisions, and consequently helping to reduce mental, emotional and physical burdens.
The m-PIMA HIV-1/2 VL joins Abbott’s comprehensive portfolio of diagnostic solutions that span the entire HIV cascade of care, serving more than 120 countries globally and all 55 countries on the African continent.
Abbott has a long and deep heritage in HIV diagnostics, having introduced the world’s first HIV test in 1985. With a leading set of solutions for screening, monitoring, management and connectivity—from the lab to the point of care, Abbott is providing critical tools to healthcare providers, especially in resource-limited settings.
The m-PIMA HIV-1/2 VL test is now commercially available in select countries. Data has been submitted for CE-IVD marking and WHO prequalification. This product is not available in the United States.
References
1 UNAIDS (2014) 90-90-90 An ambitious target to help end the AIDS epidemic. Available on http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf
2 Roberts, T., Cohn, J., Bonner, K., & Hargreaves, S. (2016). Scale-up of Routine Viral Load Testing in Resource-Poor Settings: Current and Future Implementation Challenges. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 62(8), 1043-1048. http://doi.org/10.1093/cid/ciw001