Business Wire07.10.18
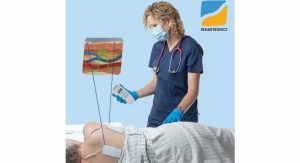
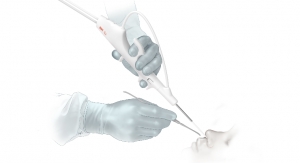
Visura Technologies, a start-up medical device company based in Evanston, Ill., has received 510(k) clearance from the US Food and Drug Administration (FDA) for its TEE Camera Assist Device (TEECAD) System. TEECAD’s single-use disposable camera easily attaches to a transesophageal echocardiogram (TEE) ultrasound probe allowing physicians to view the upper airway and esophagus during probe placement for safe intubation.
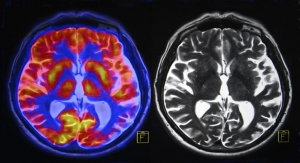
TEE is a common cardiovascular procedure, performed more than 500,000 times annually in the US, in which an ultrasound probe is placed in the patient’s esophagus to capture high-resolution ultrasound images of the heart valves and atria. Physicians currently place TEE probes blindly, guided by physician feel and patient cooperation, potentially resulting in failed intubations that can lead to cancelled therapeutic procedures and major complications such as esophageal or pharyngeal perforation.
Visura Founder Dr. David Marmor, a noninvasive cardiologist and experienced TEE operator, commented, “TEE related complications and failed intubations can be dangerous for patients and have costly ramifications for hospitals. The recent proliferation of catheter-based structural heart interventions reliant on TEE imaging has led to growth in procedural volume and an increase in the age and risk profile of patients, increasing the need for visual guidance for safe TEE probe intubation. We look forward to clinically introducing our device.”
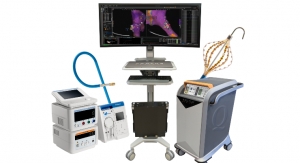
The TEECAD System consists of a single-use disposable camera Carrier that removably attaches to the TEE probe, and a separate Viewing System display that allows the physician to view real-time images from the camera to visually assist with safe probe intubation. The clearance of Visura’s first TEECAD camera Carrier is for use with the Philips X7-2t probe. The company plans to develop additional Carrier models compatible with other TEE probes available in the market.
Established in 2015, Visura Technologies is a medical device company dedicated to improving the safety and success of transesophageal echocardiography procedures.
TEE is a common cardiovascular procedure, performed more than 500,000 times annually in the US, in which an ultrasound probe is placed in the patient’s esophagus to capture high-resolution ultrasound images of the heart valves and atria. Physicians currently place TEE probes blindly, guided by physician feel and patient cooperation, potentially resulting in failed intubations that can lead to cancelled therapeutic procedures and major complications such as esophageal or pharyngeal perforation.
Visura Founder Dr. David Marmor, a noninvasive cardiologist and experienced TEE operator, commented, “TEE related complications and failed intubations can be dangerous for patients and have costly ramifications for hospitals. The recent proliferation of catheter-based structural heart interventions reliant on TEE imaging has led to growth in procedural volume and an increase in the age and risk profile of patients, increasing the need for visual guidance for safe TEE probe intubation. We look forward to clinically introducing our device.”
The TEECAD System consists of a single-use disposable camera Carrier that removably attaches to the TEE probe, and a separate Viewing System display that allows the physician to view real-time images from the camera to visually assist with safe probe intubation. The clearance of Visura’s first TEECAD camera Carrier is for use with the Philips X7-2t probe. The company plans to develop additional Carrier models compatible with other TEE probes available in the market.
Established in 2015, Visura Technologies is a medical device company dedicated to improving the safety and success of transesophageal echocardiography procedures.