PR Newswire06.06.18
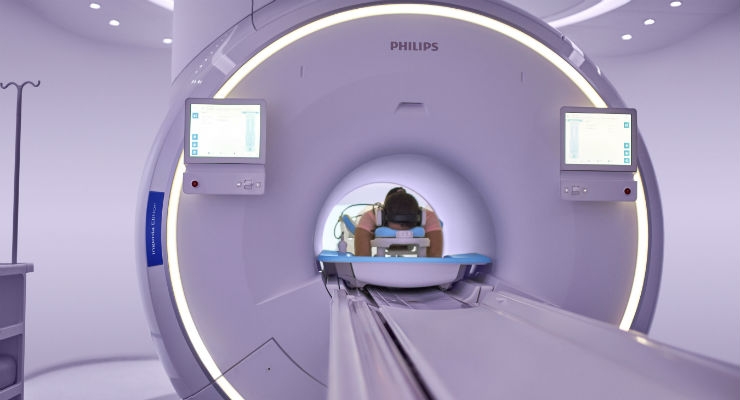
Royal Philips announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Ingenia Elition 3.0T MR solution and two clinical applications, Philips Compressed SENSE and 3D APT. This integrated suite of innovations enables clinicians to perform exams up to 50 percent faster1, increase diagnostic confidence and improve the patient experience. The first commercial installation of the Philips Ingenia Elition in the U.S. has recently been completed at Hennepin Healthcare, a comprehensive healthcare system in Minneapolis.
"Together, the Ingenia Elition and our new clinical applications make producing high-quality images fast and easy, enabling prompt diagnosis and setting the stage for effective treatment," said Arjen Radder, Global Business Leader for MR at Philips. "We're receiving a strong positive reaction from our customers as we continue to roll out our all-new Ingenia digital MR portfolio. It's providing healthcare organizations like Hennepin Health with innovative solutions that seamlessly connect data, technology and people to drive the highest quality of care."
In today's healthcare landscape—where reimbursements are becoming value-based and chronic conditions lead to more MR procedures and longer waiting times—there is an ever-increasing pressure on the radiology department. Philips is responding to these challenges through the development of new systems and clinical applications that improve image quality and the patient and staff experience, as well as operational efficiency.
"To deliver fast, consistent and accurate diagnoses, our staff need to be supported with technology that gives them the ability to provide the best patient care, in an efficient and cost-effective way," said Dr. Chip Truwit, M.D., Chair, Radiology, Hennepin Healthcare. "Philips' Ingenia Elition plays a critical role in elevating the standard of care for our patients in imaging and in improving overall operations in our new imaging center."
Improving Diagnostic Confidence and the Patient Experience
Philips Ingenia Elition and the entire digital MR portfolio empower a faster, smarter and simpler path to diagnosis—providing patients and radiologists with the following benefits:
All-New Ingenia Digital MR Portfolio
The Ingenia Elition is part of Philips' all-new Ingenia digital MR portfolio, which supports radiology departments to enhance productivity, improving the patient and staff experience, while delivering better value-based care through improved patient outcomes at lower costs. In addition to receiving U.S. FDA approval the Ingenia Elition 3.0T is also available for sale in Europe.
References
1 Using Compressed SENSE technology and compared to Philips exams without Compressed SENSE
2 SmartExam is not available to patients with MR Conditional implants
3 Togao et al. (2014) Neuro-Oncology
4 Park KJ et al. (2016) European Radiology
5 Results from case studies are not predictive of results in other cases. Results in other cases may vary
6 Compared to the average of the other five Philips MR scanners without Ambient Experience and In-bore Connect. Results from case studies are not predictive of results in other studies. Results in other cases may vary
7 Based on in-house testing
"Together, the Ingenia Elition and our new clinical applications make producing high-quality images fast and easy, enabling prompt diagnosis and setting the stage for effective treatment," said Arjen Radder, Global Business Leader for MR at Philips. "We're receiving a strong positive reaction from our customers as we continue to roll out our all-new Ingenia digital MR portfolio. It's providing healthcare organizations like Hennepin Health with innovative solutions that seamlessly connect data, technology and people to drive the highest quality of care."
In today's healthcare landscape—where reimbursements are becoming value-based and chronic conditions lead to more MR procedures and longer waiting times—there is an ever-increasing pressure on the radiology department. Philips is responding to these challenges through the development of new systems and clinical applications that improve image quality and the patient and staff experience, as well as operational efficiency.
"To deliver fast, consistent and accurate diagnoses, our staff need to be supported with technology that gives them the ability to provide the best patient care, in an efficient and cost-effective way," said Dr. Chip Truwit, M.D., Chair, Radiology, Hennepin Healthcare. "Philips' Ingenia Elition plays a critical role in elevating the standard of care for our patients in imaging and in improving overall operations in our new imaging center."
Improving Diagnostic Confidence and the Patient Experience
Philips Ingenia Elition and the entire digital MR portfolio empower a faster, smarter and simpler path to diagnosis—providing patients and radiologists with the following benefits:
- Speed of Care—By uniting Philips' unique dStream digital broadband technology with Compressed SENSE across its new digital MR portfolio, radiologists can benefit from up to 50percent1 faster exam times, increasing throughput and workflow efficiency and resulting in more time with patients. Additionally, SmartExam analytics enable automatic planning, scanning and processing of exams that help improve the entire MR workflow, from image acquisition to reading preference.
- Confident diagnosis—3D APT is a contrast-free brain MR imaging solution that addresses the need for more confident diagnosis in neuro oncology. By differentiating low-grade and high-grade gliomas, radiologists have the ability to better understand a tumor's progression and determine the effect of treatment3,4.
- Improved patient experience—The MR Elition combined with Philips Ambient Experience and in-bore Connect solution offers an immersive audio-visual experience may help calm patients, improving the quality of service for the patient. Herlev Gentofte University Hospital in Denmark managed to reduce the number of rescans by up to 70percent5 using the Ambient Experience in-bore Connect solution with its Ingenia 3.0T MR, allowing radiologists to handle more patients per day6. In addition to Ambient Experience, Philips' VitalEye brings a unique patient sensing approach, enabling an intelligent respiratory signal—allowing routine exam set-up time to occur in less than a minute7—as a part of Philips VitalScreen technology that allows workflow integration and patient setup.
All-New Ingenia Digital MR Portfolio
The Ingenia Elition is part of Philips' all-new Ingenia digital MR portfolio, which supports radiology departments to enhance productivity, improving the patient and staff experience, while delivering better value-based care through improved patient outcomes at lower costs. In addition to receiving U.S. FDA approval the Ingenia Elition 3.0T is also available for sale in Europe.
References
1 Using Compressed SENSE technology and compared to Philips exams without Compressed SENSE
2 SmartExam is not available to patients with MR Conditional implants
3 Togao et al. (2014) Neuro-Oncology
4 Park KJ et al. (2016) European Radiology
5 Results from case studies are not predictive of results in other cases. Results in other cases may vary
6 Compared to the average of the other five Philips MR scanners without Ambient Experience and In-bore Connect. Results from case studies are not predictive of results in other studies. Results in other cases may vary
7 Based on in-house testing