Abbott Laboratories05.22.18
Abbott today announced treatment with the company's Portico transcatheter aortic valve replacement (TAVR) therapy was associated with excellent clinical outcomes at 30 days, including low rates of death, disabling stroke, and paravalvular leak. Additional benefits observed included reduction in New York Heart Association (NYHA) class severity, low rates of bleeding, and improvements in a six-minute walk test, allowing patients to return to their previous lifestyles and activity faster. Data from the PORTICO I study were presented during EuroPCR, the annual meeting of the European Association of Percutaneous Cardiovascular Interventions (EAPCI), and showed favorable short-term clinical outcomes for safety and performance—consistent with the Portico CE Mark study results that granted European regulatory approval.
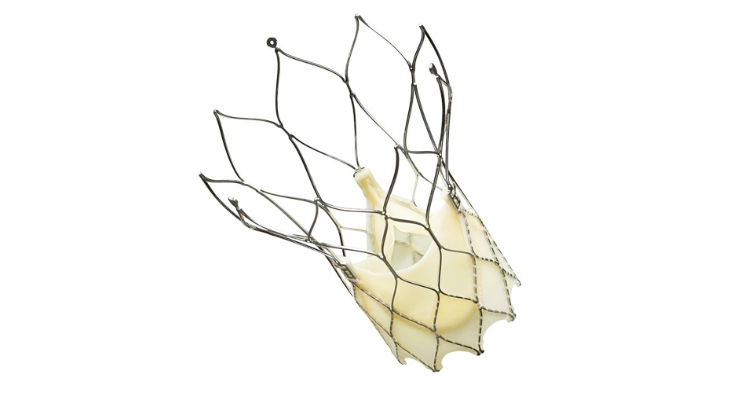
The Portico transcatheter aortic valve provides a minimally invasive treatment for patients diagnosed with severe aortic stenosis—a common and very serious valve disease—who are not candidates for open-heart surgery. More than one in eight people aged 75 and older have moderate or severe aortic stenosis,1 which is caused by a narrowing of the aortic valve opening in the heart that restricts blood flow from the left ventricle to the aorta.
Most patients don’t have symptoms for decades, but once symptoms appear, the prognosis is poor without surgical treatment.2 The end result of severe aortic valve stenosis is the reduction in the heart's pumping ability, which can lead to shortness of breath, fatigue, and ultimately to heart failure3. Transcatheter aortic valve replacement provides a minimally invasive alternative to surgical aortic valve replacement, the treatment of choice for many patients affected by severe aortic stenosis.2
"Patients with severe aortic stenosis require timely treatment to reduce symptoms and improve clinical outcomes and quality of life," said principal investigator Francesco Maisano, M.D., Prof., UniversitätsSpital Zürich, Switzerland. "These new data reinforce that treatment with the Portico valve is safe in a real-world setting—with robust clinical results—and confirm that Portico is an excellent solution for patients at increased risk for surgery."
The PORTICO I study is a real-world, multicenter, prospective, single-arm study with independent adjudication of clinical events and independent echocardiography core-lab analysis. The study will follow patients annually through five years in a real-world setting after being treated with Portico to assess long-term clinical outcomes. In the study, 941 patients were treated at 61 centers across the EU, Canada and Australia. At 30 days, the study showed patients who received Abbott's TAVR therapy had very low rates of all-cause mortality (2.7 percent; 25/941) and disabling stroke (1.7 percent; 16/941).
At 30 days after implant, study patients had excellent hemodynamic performance (8.6 mm Hg), a measurement of forward blood flow resistance, and effective orifice area, or large valve opening, of (1.8 cm2), allowing blood to easily flow from the left ventricle of the heart to the aorta. Nearly all patients (96.1 percent) experienced none-to-mild paravalvular leak which may result after a mitral or aortic valve replacement procedure. The percentage of patients in NYHA class III/IV significantly decreased from 64 percent to 13.1 percent, indicating improved mobility, and clinical measures showed a mean improvement of 26 meters in the six-minute walk test. No severe aortic valve regurgitation, or leak, based on Independent Core Lab-assessed echocardiographic analysis, was seen at 30 days.
"When developing the Portico valve, our goal was to provide physicians with new offerings that would improve deliverability, placement accuracy and performance when replacing a diseased or damaged aortic heart valve using a minimally invasive therapy," said Michael Dale, vice president of Abbott's structural heart business. "The findings of the PORTICO I real-world study corroborate those from our previous clinical trials, which showed that Portico is a life-saving valve that improves quality of life and helps patients resume their day-to-day activities."
Portico recently received regulatory approval in Europe for a sheathless introduction of the Portico valve, allowing physicians to deliver it without an arterial introducer sheath. Use of the Portico Transcatheter Aortic Valve system without an arterial delivery sheath reduces the valve profile further, making it easier for physicians to insert the device in patients with severe disease and complex anatomies. The smaller size of the valve may lower the risk of serious adverse events and vascular complications such as internal damage to the arteries.4,5,6,7
The Portico Transcatheter Aortic Valve is approved for investigational use only in the U.S.
References
1 Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11
2 Nardi P, Russo M, Saitto G, et al. The Treatment of Aortic Valve Stenosis in Patients at Intermediate-High Risk. Interv Cardiol J. 2016, 2:2.
3 Osnabrugge RLJ, Mylotte D, Head SJ, et al; Aortic Stenosis in the Elderly: Disease Prevalence and Number of Candidates for Transcatheter Aortic Valve Replacement: A Meta-Analysis and Modeling Study. Journal of the American College of Cardiology. 2013; 11:1002-1012.
4 Taramasso et al. “Feasibility and safety of Transfemoral Sheathless Portico Aortic Valve Implantation.” Catheter Cardiovasc Interv. 2017 May 13. doi: 10.1002/ccd.271005.
5 Bruschi et al. “Portico Sheathless Transcatheter Aortic Valve Implantation via Distal Axillary Artery.” Ann Thorac Surg. 2017 Feb;103(2):e175-e177. doi: 10.1016/j.athoracsur.2016.07.065
6 Denegri et al. “Real-world procedural and 30-day outcome using the Portico transcatheter aortic valve prosthesis:” Int J Cardiol. 2018 Feb 15;253:40-44. doi: 10.1016/j.ijcard.2017.10.101. Epub 2017 Nov 10
7 Taramasso M. Feasibility and Safety of Transfemoral Sheathless Portico Aortic Valve Implantation: Prelimary Results in a Single Center Experience Catheterization and Cardiovascular Interventions 91:533–539 (2018).
8 Möllmann H et al. Portico TF EU Pre-CE Mark 30d outcomes for all 4 valve sizes. J Am Coll Cardiol Intv. 2017; 10:1538–47.
The Portico transcatheter aortic valve provides a minimally invasive treatment for patients diagnosed with severe aortic stenosis—a common and very serious valve disease—who are not candidates for open-heart surgery. More than one in eight people aged 75 and older have moderate or severe aortic stenosis,1 which is caused by a narrowing of the aortic valve opening in the heart that restricts blood flow from the left ventricle to the aorta.
Most patients don’t have symptoms for decades, but once symptoms appear, the prognosis is poor without surgical treatment.2 The end result of severe aortic valve stenosis is the reduction in the heart's pumping ability, which can lead to shortness of breath, fatigue, and ultimately to heart failure3. Transcatheter aortic valve replacement provides a minimally invasive alternative to surgical aortic valve replacement, the treatment of choice for many patients affected by severe aortic stenosis.2
"Patients with severe aortic stenosis require timely treatment to reduce symptoms and improve clinical outcomes and quality of life," said principal investigator Francesco Maisano, M.D., Prof., UniversitätsSpital Zürich, Switzerland. "These new data reinforce that treatment with the Portico valve is safe in a real-world setting—with robust clinical results—and confirm that Portico is an excellent solution for patients at increased risk for surgery."
The PORTICO I study is a real-world, multicenter, prospective, single-arm study with independent adjudication of clinical events and independent echocardiography core-lab analysis. The study will follow patients annually through five years in a real-world setting after being treated with Portico to assess long-term clinical outcomes. In the study, 941 patients were treated at 61 centers across the EU, Canada and Australia. At 30 days, the study showed patients who received Abbott's TAVR therapy had very low rates of all-cause mortality (2.7 percent; 25/941) and disabling stroke (1.7 percent; 16/941).
At 30 days after implant, study patients had excellent hemodynamic performance (8.6 mm Hg), a measurement of forward blood flow resistance, and effective orifice area, or large valve opening, of (1.8 cm2), allowing blood to easily flow from the left ventricle of the heart to the aorta. Nearly all patients (96.1 percent) experienced none-to-mild paravalvular leak which may result after a mitral or aortic valve replacement procedure. The percentage of patients in NYHA class III/IV significantly decreased from 64 percent to 13.1 percent, indicating improved mobility, and clinical measures showed a mean improvement of 26 meters in the six-minute walk test. No severe aortic valve regurgitation, or leak, based on Independent Core Lab-assessed echocardiographic analysis, was seen at 30 days.
"When developing the Portico valve, our goal was to provide physicians with new offerings that would improve deliverability, placement accuracy and performance when replacing a diseased or damaged aortic heart valve using a minimally invasive therapy," said Michael Dale, vice president of Abbott's structural heart business. "The findings of the PORTICO I real-world study corroborate those from our previous clinical trials, which showed that Portico is a life-saving valve that improves quality of life and helps patients resume their day-to-day activities."
Portico recently received regulatory approval in Europe for a sheathless introduction of the Portico valve, allowing physicians to deliver it without an arterial introducer sheath. Use of the Portico Transcatheter Aortic Valve system without an arterial delivery sheath reduces the valve profile further, making it easier for physicians to insert the device in patients with severe disease and complex anatomies. The smaller size of the valve may lower the risk of serious adverse events and vascular complications such as internal damage to the arteries.4,5,6,7
The Portico Transcatheter Aortic Valve is approved for investigational use only in the U.S.
References
1 Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11
2 Nardi P, Russo M, Saitto G, et al. The Treatment of Aortic Valve Stenosis in Patients at Intermediate-High Risk. Interv Cardiol J. 2016, 2:2.
3 Osnabrugge RLJ, Mylotte D, Head SJ, et al; Aortic Stenosis in the Elderly: Disease Prevalence and Number of Candidates for Transcatheter Aortic Valve Replacement: A Meta-Analysis and Modeling Study. Journal of the American College of Cardiology. 2013; 11:1002-1012.
4 Taramasso et al. “Feasibility and safety of Transfemoral Sheathless Portico Aortic Valve Implantation.” Catheter Cardiovasc Interv. 2017 May 13. doi: 10.1002/ccd.271005.
5 Bruschi et al. “Portico Sheathless Transcatheter Aortic Valve Implantation via Distal Axillary Artery.” Ann Thorac Surg. 2017 Feb;103(2):e175-e177. doi: 10.1016/j.athoracsur.2016.07.065
6 Denegri et al. “Real-world procedural and 30-day outcome using the Portico transcatheter aortic valve prosthesis:” Int J Cardiol. 2018 Feb 15;253:40-44. doi: 10.1016/j.ijcard.2017.10.101. Epub 2017 Nov 10
7 Taramasso M. Feasibility and Safety of Transfemoral Sheathless Portico Aortic Valve Implantation: Prelimary Results in a Single Center Experience Catheterization and Cardiovascular Interventions 91:533–539 (2018).
8 Möllmann H et al. Portico TF EU Pre-CE Mark 30d outcomes for all 4 valve sizes. J Am Coll Cardiol Intv. 2017; 10:1538–47.