Business Wire04.19.18
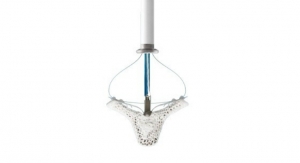
Motus GI Holdings Inc., a medical technology company dedicated to improving endoscopy outcomes and experiences, announced that the European Patent Office (EPO) has issued a patent No. 3079556 related to the Pure-Vu System, a medical device that cleans the colon to facilitate improved visualization during a colonoscopy procedure to enable a quality exam.
“This European patent strengthens our intellectual property and confirms the Pure-Vu System’s place as an important proprietary technology in the European market,” said Mark Pomeranz, CEO of Motus. “Combined with our recent European regulatory approval, this patent will enable us to establish a strong presence in Europe, where 6 million colonoscopies are performed annually.”
The Pure-Vu System is currently being introduced on a pilot basis in the U.S. market. Motus is planning to initiate a full commercial launch in the United States and select international markets in 2019. The company recently announced CE mark approval for the Pure-Vu System in Europe and expects to continue to involve key European clinical centers in the post-approval clinical trials that it plans to conduct during the next 12 months and beyond.
“With 30 million colonoscopy procedures performed annually worldwide, there is tremendous potential for the Pure-Vu System to improve clinical outcomes and reduce costs. Specifically, of the nearly 4 million in-patient colonoscopy procedures performed every year, where nearly 50 percent of patients present with an inadequately prepped colon. This contributes to long procedure times, repeat procedures, poor detection rates and extended hospital stays, all of which we believe the Pure-Vu System can help address by effectively and efficiently cleansing the colon during the procedure,” Pomeranz added.
The Pure-Vu System is a 510(k) U.S. Food and Drug Administration-cleared medical device indicated to help facilitate the cleaning of a poorly prepared colon during the colonoscopy procedure. The device integrates with standard colonoscopes to enable cleaning during the procedure while preserving standard procedural workflow and techniques. The Pure-Vu System has received CE mark approval in Europe.
Motus GI Holdings Inc. is a medical technology company, with subsidiaries in the United States and Israel, dedicated to improving endoscopy outcomes, lowering costs and enhancing patient experiences. The company is focused on the development and commercialization of the Pure-Vu System to improve the colonoscopy experience and assist in the early detection and prevention of colorectal cancer and other diseases of the rectum and colon.
“This European patent strengthens our intellectual property and confirms the Pure-Vu System’s place as an important proprietary technology in the European market,” said Mark Pomeranz, CEO of Motus. “Combined with our recent European regulatory approval, this patent will enable us to establish a strong presence in Europe, where 6 million colonoscopies are performed annually.”
The Pure-Vu System is currently being introduced on a pilot basis in the U.S. market. Motus is planning to initiate a full commercial launch in the United States and select international markets in 2019. The company recently announced CE mark approval for the Pure-Vu System in Europe and expects to continue to involve key European clinical centers in the post-approval clinical trials that it plans to conduct during the next 12 months and beyond.
“With 30 million colonoscopy procedures performed annually worldwide, there is tremendous potential for the Pure-Vu System to improve clinical outcomes and reduce costs. Specifically, of the nearly 4 million in-patient colonoscopy procedures performed every year, where nearly 50 percent of patients present with an inadequately prepped colon. This contributes to long procedure times, repeat procedures, poor detection rates and extended hospital stays, all of which we believe the Pure-Vu System can help address by effectively and efficiently cleansing the colon during the procedure,” Pomeranz added.
The Pure-Vu System is a 510(k) U.S. Food and Drug Administration-cleared medical device indicated to help facilitate the cleaning of a poorly prepared colon during the colonoscopy procedure. The device integrates with standard colonoscopes to enable cleaning during the procedure while preserving standard procedural workflow and techniques. The Pure-Vu System has received CE mark approval in Europe.
Motus GI Holdings Inc. is a medical technology company, with subsidiaries in the United States and Israel, dedicated to improving endoscopy outcomes, lowering costs and enhancing patient experiences. The company is focused on the development and commercialization of the Pure-Vu System to improve the colonoscopy experience and assist in the early detection and prevention of colorectal cancer and other diseases of the rectum and colon.