U.S. Food and Drug Administration03.23.18
The FDA has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death.
Recalled Product:
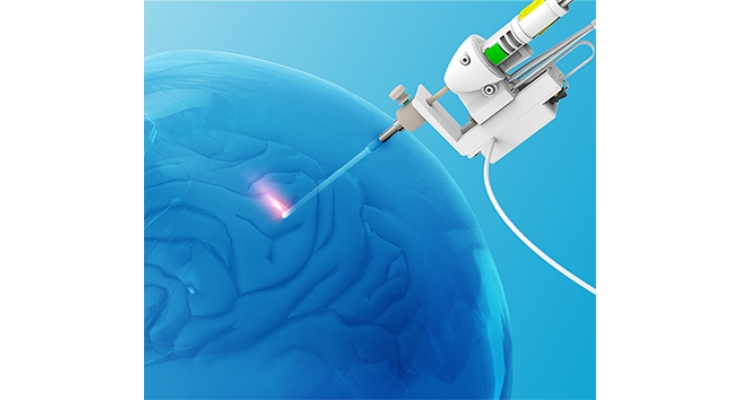
NeuroBlate Laser Delivery Probes are small, carbon dioxide (CO2)-cooled catheters that allow minimally invasive entry into a patient’s brain. The probes are part of the Monteris Medical NeuroBlate System, which is used during surgical procedures to remove (ablate), thicken or solidify (coagulate), or destroy (necrotize) cells in brain tissue.
In some cases, the NeuroBlate Laser Delivery Probes interact with the MRI system used to visualize the position of the catheter and cause unexpected heating and damage to the tip of the probe. This could cause unanticipated heating of surrounding brain tissue, or damage the tip of the probe, and allow the CO2 cooling gas inside the probe to leak into the brain.
Until appropriate mitigation strategies have been identified by the manufacturer and evaluated by the FDA, the FDA recommends health care providers should strongly consider treating patients using alternative procedures if available. Health care providers who do not believe there is a viable alternative should use the device with extreme caution.
Patients who need to undergo brain tissue ablation and neurosurgeons who may be using these devices may be affected by this recall.
Monteris has issued three product advisories between October and December 2017 related to this issue; however the FDA has concerns that the information provided by Monteris has not sufficiently mitigated the risk of unintended probe tip heating.
Until appropriate mitigation strategies have been identified by the manufacturer and evaluated by the FDA, health care providers should strongly consider treating patients using alternative procedures if available. The benefits and risks of the device, as well as the availability and benefits and risks of alternative treatment modalities, should be considered on an individual patient basis. Healthcare providers who do not believe there is a viable alternative should use the device with extreme caution.
For more information, read the Letter to Health Care Providers issued by the FDA on March 22, 2018.
Recalled Product:
- Name: Monteris Medical NeuroBlate System and Laser Delivery Probes
- Product codes: GEX, HAW
- Models: SideFire 3.3 mm (SFP) Directional Laser Probe (sizes 000-5), FullFire 3.3 mm DTP Diffusing Tip Laser Probe (sizes 000-5), FullFire Select 2.2 mm Diffusing Tip Laser Probe (sizes 000-5)
- Manufacturing and Distribution Dates: April 2013 to July 2017
- Number of Affected Devices: 52 (49 in the US, 3 in Canada)
NeuroBlate Laser Delivery Probes are small, carbon dioxide (CO2)-cooled catheters that allow minimally invasive entry into a patient’s brain. The probes are part of the Monteris Medical NeuroBlate System, which is used during surgical procedures to remove (ablate), thicken or solidify (coagulate), or destroy (necrotize) cells in brain tissue.
In some cases, the NeuroBlate Laser Delivery Probes interact with the MRI system used to visualize the position of the catheter and cause unexpected heating and damage to the tip of the probe. This could cause unanticipated heating of surrounding brain tissue, or damage the tip of the probe, and allow the CO2 cooling gas inside the probe to leak into the brain.
Until appropriate mitigation strategies have been identified by the manufacturer and evaluated by the FDA, the FDA recommends health care providers should strongly consider treating patients using alternative procedures if available. Health care providers who do not believe there is a viable alternative should use the device with extreme caution.
Patients who need to undergo brain tissue ablation and neurosurgeons who may be using these devices may be affected by this recall.
Monteris has issued three product advisories between October and December 2017 related to this issue; however the FDA has concerns that the information provided by Monteris has not sufficiently mitigated the risk of unintended probe tip heating.
Until appropriate mitigation strategies have been identified by the manufacturer and evaluated by the FDA, health care providers should strongly consider treating patients using alternative procedures if available. The benefits and risks of the device, as well as the availability and benefits and risks of alternative treatment modalities, should be considered on an individual patient basis. Healthcare providers who do not believe there is a viable alternative should use the device with extreme caution.
For more information, read the Letter to Health Care Providers issued by the FDA on March 22, 2018.