American College of Cardiology03.12.18
At two years of follow-up, severely ill patients with advanced heart failure who received a novel heart pump fully implantable within the chest experienced no malfunctions requiring replacement or removal of the device for blood clotting. Further, their risk of a stroke was halved compared with patients who received the established version of the pump that requires an abdominal location for the implant, according to research presented at the American College of Cardiology’s 67th Annual Scientific Session.
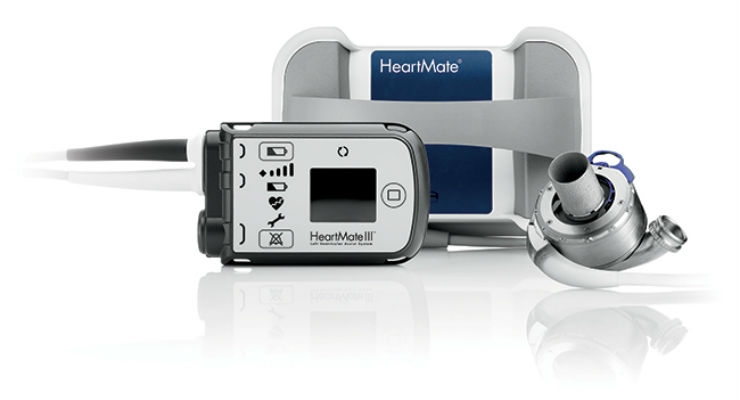
The new device tested in this trial, the HeartMate 3, is the first implantable mechanical heart pump, or left ventricular assist device (LVAD), to use fully magnetic levitation technology—which makes the pump frictionless, or without mechanical bearings—to push blood through the device and into the aorta, the body’s central artery, said Mandeep R. Mehra, M.D., medical director of the Heart and Vascular Center, Brigham and Women’s Hospital in Boston and lead author of the study.
“We saw a marked decrease in pump malfunction requiring reoperation, mostly driven by an absence of confirmed pump thrombosis, and a halving of observed stroke rates in patients implanted with the HeartMate 3 compared with patients who were implanted with the HeartMate II,” Mehra said.
Although the trial was designed to show only that outcomes with the HeartMate 3 were no worse than those with the HeartMate II, the results demonstrate that outcomes are, in fact, clearly superior with the HeartMate 3 than with the standard device, he said.
In advanced heart failure, the heart’s main pumping chamber, the left ventricle, is too weak to pump oxygen-rich blood from the lungs throughout the body. An LVAD is designed to supplement the pumping ability of the weakened heart in late-stage heart failure. It is implanted next to the heart and attached to the aorta, the main artery that sends blood to the rest of the body. The device is attached to and powered by an external battery pack worn by the patient.
The trial, known as MOMENTUM-3, enrolled 1,028 patients at 69 centers in the U.S. Patients’ median age was 60 years and 78 percent were men. All had severe heart failure that left them unable to engage in any physical activity without discomfort. Most had symptoms of fatigue or shortness of breath even when resting. Most (85 percent) were receiving intravenous heart failure medication because pills alone no longer worked or caused intolerable adverse effects.
Some patients in the study needed an LVAD to sustain them until they were able to receive a heart transplant. Others, because of age or other health problems, were not candidates for a transplant and relied on an LVAD as lifelong therapy.
Patients were randomly assigned to be surgically implanted with either a HeartMate II or a HeartMate 3. All patients received blood-thinning medications following surgery. The primary study endpoint was the combined rate of disabling stroke, device malfunction requiring surgery to replace or remove it, and death from heart failure. Six-month outcomes for 294 patients were reported in the New England Journal of Medicine in November 2016. The current study reports outcomes for 366 patients who have completed two years of follow-up.
A total of 82.8 percent of HeartMate 3 patients were alive at two years, compared with 76.2 percent of HeartMate II patients, Mehra said. One percent of HeartMate 3 patients needed additional surgery to remove or replace the device (due to an electrical or mechanical malfunction), compared with 17 percent of HeartMate II patients (two-thirds of which were for pump clotting). HeartMate 3 patients were also less likely to have a stroke, occurring in 10 percent vs. 19 percent of HeartMate II patients. However, the rate of disabling stroke—defined as a stroke resulting in severe inability to walk or attend to bodily needs without assistance—was not significantly different in the two groups of patients.
“All of the benefit seen with the HeartMate 3 was in reducing the rate of nondisabling strokes,” Mehra said. “Disabling stroke was a low-frequency event—occurring in 5 to 7 percent of all patients in the trial. The overall stroke rate at two years is the lowest recorded to date in an LVAD trial.”
One limitation of the current study is that it was impossible for either patients or their doctors to be blinded to which device was implanted, Mehra said. The lack of blinding could have led to differences in the way doctors managed patients who had received the HeartMate 3 compared with the HeartMate II. However, the trial was rigorously designed to prevent such differences from occurring, he said.
Another potential limitation is that the surgical techniques for implanting the two devices are different. All centers participating in the study had considerable experience with the HeartMate II and all received the same training on how to implant the HeartMate 3.
In addition to its use of magnetic levitation technology, the HeartMate 3 device has other design features that distinguish it from the HeartMate II, Mehra said.
“First, the HeartMate 3 is small enough to be fully implanted in the chest, whereas the HeartMate II requires a ‘pocket’ to be created in the abdomen to accommodate the device,” he said. “Second, the blood-flow pathway is wider in the HeartMate 3, which may contribute to the reduction in pump thrombosis. Third, the HeartMate 3 is designed to speed up and slow down every two seconds, creating an artificial fixed pulse that may prevent blood from building up inside the device.”
The HeartMate 3 received FDA approval on Aug. 31, 2017, for use in patients with advanced heart failure who are awaiting a heart transplant.
Innovative features in the design of the MOMENTUM-3 trial made it possible to obtain initial FDA approval of the HeartMate 3 in under three years, Mehra said, in contrast to the approximately seven years that this process has typically taken in the past. Among these innovations is the decision to enroll patients whether or not they were candidates for a heart transplant, he said. In the past, an initial trial would have assessed the safety of the device, a second trial would have tested it in patients awaiting a heart transplant and a third trial would have examined patients ineligible for a transplant.
Two-year follow-up results for all 1,028 patients enrolled in the trial are expected by late 2019, he said.
The study was funded by Abbott Inc.
The new device tested in this trial, the HeartMate 3, is the first implantable mechanical heart pump, or left ventricular assist device (LVAD), to use fully magnetic levitation technology—which makes the pump frictionless, or without mechanical bearings—to push blood through the device and into the aorta, the body’s central artery, said Mandeep R. Mehra, M.D., medical director of the Heart and Vascular Center, Brigham and Women’s Hospital in Boston and lead author of the study.
“We saw a marked decrease in pump malfunction requiring reoperation, mostly driven by an absence of confirmed pump thrombosis, and a halving of observed stroke rates in patients implanted with the HeartMate 3 compared with patients who were implanted with the HeartMate II,” Mehra said.
Although the trial was designed to show only that outcomes with the HeartMate 3 were no worse than those with the HeartMate II, the results demonstrate that outcomes are, in fact, clearly superior with the HeartMate 3 than with the standard device, he said.
In advanced heart failure, the heart’s main pumping chamber, the left ventricle, is too weak to pump oxygen-rich blood from the lungs throughout the body. An LVAD is designed to supplement the pumping ability of the weakened heart in late-stage heart failure. It is implanted next to the heart and attached to the aorta, the main artery that sends blood to the rest of the body. The device is attached to and powered by an external battery pack worn by the patient.
The trial, known as MOMENTUM-3, enrolled 1,028 patients at 69 centers in the U.S. Patients’ median age was 60 years and 78 percent were men. All had severe heart failure that left them unable to engage in any physical activity without discomfort. Most had symptoms of fatigue or shortness of breath even when resting. Most (85 percent) were receiving intravenous heart failure medication because pills alone no longer worked or caused intolerable adverse effects.
Some patients in the study needed an LVAD to sustain them until they were able to receive a heart transplant. Others, because of age or other health problems, were not candidates for a transplant and relied on an LVAD as lifelong therapy.
Patients were randomly assigned to be surgically implanted with either a HeartMate II or a HeartMate 3. All patients received blood-thinning medications following surgery. The primary study endpoint was the combined rate of disabling stroke, device malfunction requiring surgery to replace or remove it, and death from heart failure. Six-month outcomes for 294 patients were reported in the New England Journal of Medicine in November 2016. The current study reports outcomes for 366 patients who have completed two years of follow-up.
A total of 82.8 percent of HeartMate 3 patients were alive at two years, compared with 76.2 percent of HeartMate II patients, Mehra said. One percent of HeartMate 3 patients needed additional surgery to remove or replace the device (due to an electrical or mechanical malfunction), compared with 17 percent of HeartMate II patients (two-thirds of which were for pump clotting). HeartMate 3 patients were also less likely to have a stroke, occurring in 10 percent vs. 19 percent of HeartMate II patients. However, the rate of disabling stroke—defined as a stroke resulting in severe inability to walk or attend to bodily needs without assistance—was not significantly different in the two groups of patients.
“All of the benefit seen with the HeartMate 3 was in reducing the rate of nondisabling strokes,” Mehra said. “Disabling stroke was a low-frequency event—occurring in 5 to 7 percent of all patients in the trial. The overall stroke rate at two years is the lowest recorded to date in an LVAD trial.”
One limitation of the current study is that it was impossible for either patients or their doctors to be blinded to which device was implanted, Mehra said. The lack of blinding could have led to differences in the way doctors managed patients who had received the HeartMate 3 compared with the HeartMate II. However, the trial was rigorously designed to prevent such differences from occurring, he said.
Another potential limitation is that the surgical techniques for implanting the two devices are different. All centers participating in the study had considerable experience with the HeartMate II and all received the same training on how to implant the HeartMate 3.
In addition to its use of magnetic levitation technology, the HeartMate 3 device has other design features that distinguish it from the HeartMate II, Mehra said.
“First, the HeartMate 3 is small enough to be fully implanted in the chest, whereas the HeartMate II requires a ‘pocket’ to be created in the abdomen to accommodate the device,” he said. “Second, the blood-flow pathway is wider in the HeartMate 3, which may contribute to the reduction in pump thrombosis. Third, the HeartMate 3 is designed to speed up and slow down every two seconds, creating an artificial fixed pulse that may prevent blood from building up inside the device.”
The HeartMate 3 received FDA approval on Aug. 31, 2017, for use in patients with advanced heart failure who are awaiting a heart transplant.
Innovative features in the design of the MOMENTUM-3 trial made it possible to obtain initial FDA approval of the HeartMate 3 in under three years, Mehra said, in contrast to the approximately seven years that this process has typically taken in the past. Among these innovations is the decision to enroll patients whether or not they were candidates for a heart transplant, he said. In the past, an initial trial would have assessed the safety of the device, a second trial would have tested it in patients awaiting a heart transplant and a third trial would have examined patients ineligible for a transplant.
Two-year follow-up results for all 1,028 patients enrolled in the trial are expected by late 2019, he said.
The study was funded by Abbott Inc.