Business Wire02.20.18
AngioDynamics Inc., a provider of minimally invasive medical devices for vascular access, peripheral vascular disease, surgery, and oncology, announced that the U.S. Food and Drug Administration (FDA) has granted the Expedited Access Pathway (EAP) designation to the company’s NanoKnife System and proposed indication for use for the treatment of Stage III pancreatic cancer.
The EAP program is designed to help patients gain more timely access to medical devices that may provide more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions, for which no approved or cleared alternatives exist. This is achieved by expediting the device’s assessment and review processes through more interactive and timely communication with the FDA, pre- and post-market balance of data collection requirements, efficient and flexible clinical study design, FDA review team support and Agency senior management engagement, and priority review.
Pursuant to the recently enacted 21st Century Cures Act, the FDA has released draft guidance for a Breakthrough Devices Program, which, when finalized, will supersede the EAP. The FDA has indicated that all devices that receive EAP designation will gain Breakthrough Device designation when the guidance document becomes final.
Jim Clemmer, President and CEO of AngioDynamics, commented, “The Expedited Access Pathway and Breakthrough Devices Program is an important and meaningful initiative to prioritize review and approval for novel, innovative devices needed by patients for the treatment of life-threatening diseases and conditions. We are thrilled that the FDA has granted the EAP designation to NanoKnife for the treatment of Stage III pancreatic cancer and are excited to continue working with the FDA toward approval of NanoKnife as a treatment for the underserved patient population suffering from this deadly disease.”
According to the American Cancer Society, pancreatic cancer is the third leading cause of cancer related deaths in the United States and is projected to increase to the second leading cause within the next five years. There are over 55,000 new cases and 44,000 estimated deaths in the US annually.1 The mortality rate is high due to the aggressive nature of the disease and lack of early warning signs. In fact, only 20 percent to 30 percent of patients are candidates for surgical resection at time of diagnosis.2 Approximately 40 percent percent of patients will present with Stage III and 40 percent with metastatic disease. Regardless of the stage of pancreatic cancer, it has the lowest survival rate of any cancer, with an overall one- and five-year survival rate of 27 percent and 8 percent, respectively.1
There are limited treatment options for Stage III and IV disease, with chemotherapy and/or radiotherapy considered the standard of care.2 There have been advancements in both techniques, but this has come at the cost of greater toxicity,3 limiting the patients that are candidates for the treatment.
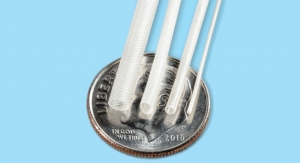
The NanoKnife System has received 510(k) clearance from the FDA for the surgical ablation of soft tissue. The NanoKnife Ablation System utilizes low energy direct current electrical pulses to permanently open pores in target cell membranes. These permanent pores or nano-scale defects in the cell membranes result in cell death. The treated tissue is then removed by the body’s natural processes in a matter of weeks, mimicking natural cell death. Unlike other ablation technologies, NanoKnife Ablation System does not achieve tissue ablation using thermal energy.
The NanoKnife Ablation System consists of two major components: a Low Energy Direct Current, or LEDC Generator and needle-like electrode probes. Up to six electrode probes can be placed into or around the targeted soft tissue. Once the probes are in place, the user enters the appropriate parameters for voltage, number of pulses, interval between pulses, and the pulse length into the generator user interface. The generator then delivers a series of short electric pulses between each electrode probe. The energy delivery is hyperechoic and can be monitored under real-time ultrasound.
AngioDynamics provides minimally invasive medical devices used by professional healthcare providers for vascular access, surgery, peripheral vascular disease, and oncology. AngioDynamics’ diverse product lines include ablation systems, fluid management systems, vascular access products, angiographic products and accessories drainage products, thrombolytic products and venous products. In the United States, the NanoKnife System has received a 510(k) clearance by the Food and Drug Administration for use in the surgical ablation of soft tissue, and is similarly approved for commercialization in Canada, the European Union and Australia. The NanoKnife System has not been cleared for the treatment or therapy of a specific disease or condition.
References
1. CA Cancer J Clin. 2018 Jan;68(1):7-30. doi: 10.3322/caac.21442. Epub 2018 Jan 4.
2. J Natl Compr Canc Netw. 2017 Aug;15(8):1028-1061. doi: 10.6004/jnccn.2017.0131.
3. N Engl J Med. 2011 May 12;364(19):1817-25. doi: 10.1056/NEJMoa1011923.
The EAP program is designed to help patients gain more timely access to medical devices that may provide more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions, for which no approved or cleared alternatives exist. This is achieved by expediting the device’s assessment and review processes through more interactive and timely communication with the FDA, pre- and post-market balance of data collection requirements, efficient and flexible clinical study design, FDA review team support and Agency senior management engagement, and priority review.
Pursuant to the recently enacted 21st Century Cures Act, the FDA has released draft guidance for a Breakthrough Devices Program, which, when finalized, will supersede the EAP. The FDA has indicated that all devices that receive EAP designation will gain Breakthrough Device designation when the guidance document becomes final.
Jim Clemmer, President and CEO of AngioDynamics, commented, “The Expedited Access Pathway and Breakthrough Devices Program is an important and meaningful initiative to prioritize review and approval for novel, innovative devices needed by patients for the treatment of life-threatening diseases and conditions. We are thrilled that the FDA has granted the EAP designation to NanoKnife for the treatment of Stage III pancreatic cancer and are excited to continue working with the FDA toward approval of NanoKnife as a treatment for the underserved patient population suffering from this deadly disease.”
According to the American Cancer Society, pancreatic cancer is the third leading cause of cancer related deaths in the United States and is projected to increase to the second leading cause within the next five years. There are over 55,000 new cases and 44,000 estimated deaths in the US annually.1 The mortality rate is high due to the aggressive nature of the disease and lack of early warning signs. In fact, only 20 percent to 30 percent of patients are candidates for surgical resection at time of diagnosis.2 Approximately 40 percent percent of patients will present with Stage III and 40 percent with metastatic disease. Regardless of the stage of pancreatic cancer, it has the lowest survival rate of any cancer, with an overall one- and five-year survival rate of 27 percent and 8 percent, respectively.1
There are limited treatment options for Stage III and IV disease, with chemotherapy and/or radiotherapy considered the standard of care.2 There have been advancements in both techniques, but this has come at the cost of greater toxicity,3 limiting the patients that are candidates for the treatment.
The NanoKnife System has received 510(k) clearance from the FDA for the surgical ablation of soft tissue. The NanoKnife Ablation System utilizes low energy direct current electrical pulses to permanently open pores in target cell membranes. These permanent pores or nano-scale defects in the cell membranes result in cell death. The treated tissue is then removed by the body’s natural processes in a matter of weeks, mimicking natural cell death. Unlike other ablation technologies, NanoKnife Ablation System does not achieve tissue ablation using thermal energy.
The NanoKnife Ablation System consists of two major components: a Low Energy Direct Current, or LEDC Generator and needle-like electrode probes. Up to six electrode probes can be placed into or around the targeted soft tissue. Once the probes are in place, the user enters the appropriate parameters for voltage, number of pulses, interval between pulses, and the pulse length into the generator user interface. The generator then delivers a series of short electric pulses between each electrode probe. The energy delivery is hyperechoic and can be monitored under real-time ultrasound.
AngioDynamics provides minimally invasive medical devices used by professional healthcare providers for vascular access, surgery, peripheral vascular disease, and oncology. AngioDynamics’ diverse product lines include ablation systems, fluid management systems, vascular access products, angiographic products and accessories drainage products, thrombolytic products and venous products. In the United States, the NanoKnife System has received a 510(k) clearance by the Food and Drug Administration for use in the surgical ablation of soft tissue, and is similarly approved for commercialization in Canada, the European Union and Australia. The NanoKnife System has not been cleared for the treatment or therapy of a specific disease or condition.
References
1. CA Cancer J Clin. 2018 Jan;68(1):7-30. doi: 10.3322/caac.21442. Epub 2018 Jan 4.
2. J Natl Compr Canc Netw. 2017 Aug;15(8):1028-1061. doi: 10.6004/jnccn.2017.0131.
3. N Engl J Med. 2011 May 12;364(19):1817-25. doi: 10.1056/NEJMoa1011923.