Boston Scientific12.12.17
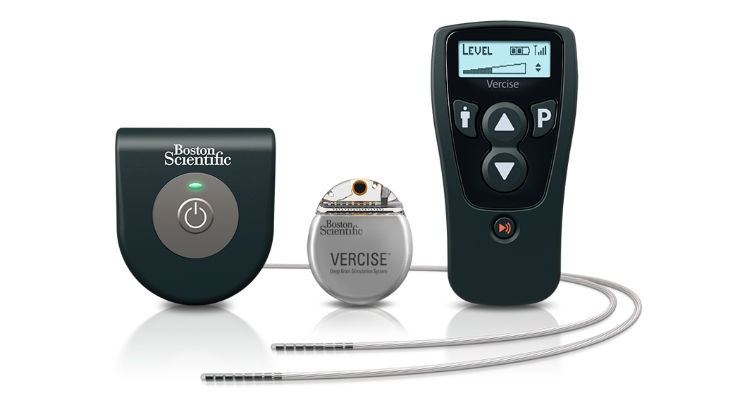
Boston Scientific Corporation announced that it has received approval from the U.S. Food and Drug Administration (FDA) for the Vercise Deep Brain Stimulation (DBS) System. DBS is used to treat the symptoms of Parkinson’s disease (PD), a degenerative condition that affects more than one million people in the United States and 10 million worldwide.1 DBS works by stimulating a targeted region of the brain through implanted leads that are powered by a device called an implantable pulse generator (IPG).
The approval was based on the INTREPID study, the first multi-center, prospective, double-blind, randomized sham-controlled study of DBS for PD in the U.S. The INTREPID study evaluated the safety of the system in 292 patients at 23 sites and also evaluated its effectiveness. It successfully met its primary endpoint of mean change in waking hours with good symptom control (n=160). Data from the INTREPID study is expected to be released in 2018. The filing was also supported by safety data from the European multi-center, prospective, single-arm VANTAGE study. In the VANTAGE study, 40 patients treated with the Vercise DBS System demonstrated a 63 percent improvement in motor function at 52 weeks from baseline as measured by the Unified Parkinson’s Disease Rating Scale III, as well as improvements in quality of life and medication usage.2
Following FDA approval, the first commercial implant in the U.S. with the Vercise System will take place at the University of Minnesota Medical Center in Minneapolis, MN by faculty physicians from the University of Minnesota Medical School including Jerry Vitek, M.D., Ph.D, professor and chair, Department of Neurology, Michael C. Park, M.D., Ph.D, assistant professor, Department of Neurosurgery and Lauren E. Schrock, M.D., MnDrive neuromodulation faculty scholar, Department of Neurology.
“The Vercise DBS System changes the landscape of what physicians can do to help improve the quality of life for people living with Parkinson’s disease,” said Dr. Vitek, coordinating principal investigator for the INTREPID study. “This system provides an ability to sculpt the current field in the DBS target using novel technology that offers flexibility in programming. This flexibility allows us to target different regions of the subthalamic nucleus, which we believe will improve outcomes while reducing side effects.”
The Vercise System first launched in Europe in 2012 and was developed from a foundation of cochlear implant technology, designed to specifically stimulate auditory nerves to produce a sense of hearing. The Vercise IPG is the smallest, rechargeable DBS device available in the U.S. and, depending on individual use, can have a battery life of more than 15 years. The Vercise System has a proprietary capability that independently controls the amount of current delivered by each of the electrodes on the implanted leads. The Vercise System leads have eight contacts with a long total span of 15.5mm, tight spacing between each contact of .5mm, and multi-lumen construction. These system features are designed to work together to address common challenges in DBS therapy such as fluctuations in symptoms and the progressive nature of the condition by offering more adaptable delivery of stimulation.
“This approval marks an important step for patients who will now have the choice to be treated with one of the most innovative neuromodulation technologies available today,” said Maulik Nanavaty, president and senior vice president, Neuromodulation, Boston Scientific. “Our system stands apart from the field in its approach and is changing the traditional definition on how we can leverage technology to treat patients with Parkinson’s disease.”
Since 2012, Boston Scientific has continued to advance its DBS portfolio, including the launch this year in Europe of the Vercise Gevia Deep Brain Stimulation System with the Cartesia Lead, providing the first directional, rechargeable and magnetic resonance (MR) conditional system.3 The company also recently introduced a new programming software, Neural Navigator 2, which enables physicians to visualize the stimulation field while configuring DBS programs for patients. The company is also supporting ongoing research in the field, including the use of DBS for stroke recovery and to treat Alzheimer’s disease.
References
1http://www.pdf.org/parkinson_statistics Accessed 04AUG2017.
2Timmermann, Lars et al. Multiple-source current steering in subthalamic nucleus deep brain stimulation for Parkinson’s disease (the VANTAGE study): a non-randomised, prospective, multicentre, open-label study. The Lancet Neurology, Volume 14, Issue 7, 693 - 701.
31.5 Tesla MR conditional when all conditions of use are met.
The approval was based on the INTREPID study, the first multi-center, prospective, double-blind, randomized sham-controlled study of DBS for PD in the U.S. The INTREPID study evaluated the safety of the system in 292 patients at 23 sites and also evaluated its effectiveness. It successfully met its primary endpoint of mean change in waking hours with good symptom control (n=160). Data from the INTREPID study is expected to be released in 2018. The filing was also supported by safety data from the European multi-center, prospective, single-arm VANTAGE study. In the VANTAGE study, 40 patients treated with the Vercise DBS System demonstrated a 63 percent improvement in motor function at 52 weeks from baseline as measured by the Unified Parkinson’s Disease Rating Scale III, as well as improvements in quality of life and medication usage.2
Following FDA approval, the first commercial implant in the U.S. with the Vercise System will take place at the University of Minnesota Medical Center in Minneapolis, MN by faculty physicians from the University of Minnesota Medical School including Jerry Vitek, M.D., Ph.D, professor and chair, Department of Neurology, Michael C. Park, M.D., Ph.D, assistant professor, Department of Neurosurgery and Lauren E. Schrock, M.D., MnDrive neuromodulation faculty scholar, Department of Neurology.
“The Vercise DBS System changes the landscape of what physicians can do to help improve the quality of life for people living with Parkinson’s disease,” said Dr. Vitek, coordinating principal investigator for the INTREPID study. “This system provides an ability to sculpt the current field in the DBS target using novel technology that offers flexibility in programming. This flexibility allows us to target different regions of the subthalamic nucleus, which we believe will improve outcomes while reducing side effects.”
The Vercise System first launched in Europe in 2012 and was developed from a foundation of cochlear implant technology, designed to specifically stimulate auditory nerves to produce a sense of hearing. The Vercise IPG is the smallest, rechargeable DBS device available in the U.S. and, depending on individual use, can have a battery life of more than 15 years. The Vercise System has a proprietary capability that independently controls the amount of current delivered by each of the electrodes on the implanted leads. The Vercise System leads have eight contacts with a long total span of 15.5mm, tight spacing between each contact of .5mm, and multi-lumen construction. These system features are designed to work together to address common challenges in DBS therapy such as fluctuations in symptoms and the progressive nature of the condition by offering more adaptable delivery of stimulation.
“This approval marks an important step for patients who will now have the choice to be treated with one of the most innovative neuromodulation technologies available today,” said Maulik Nanavaty, president and senior vice president, Neuromodulation, Boston Scientific. “Our system stands apart from the field in its approach and is changing the traditional definition on how we can leverage technology to treat patients with Parkinson’s disease.”
Since 2012, Boston Scientific has continued to advance its DBS portfolio, including the launch this year in Europe of the Vercise Gevia Deep Brain Stimulation System with the Cartesia Lead, providing the first directional, rechargeable and magnetic resonance (MR) conditional system.3 The company also recently introduced a new programming software, Neural Navigator 2, which enables physicians to visualize the stimulation field while configuring DBS programs for patients. The company is also supporting ongoing research in the field, including the use of DBS for stroke recovery and to treat Alzheimer’s disease.
References
1http://www.pdf.org/parkinson_statistics Accessed 04AUG2017.
2Timmermann, Lars et al. Multiple-source current steering in subthalamic nucleus deep brain stimulation for Parkinson’s disease (the VANTAGE study): a non-randomised, prospective, multicentre, open-label study. The Lancet Neurology, Volume 14, Issue 7, 693 - 701.
31.5 Tesla MR conditional when all conditions of use are met.