Business Wire07.28.17
W. L. Gore & Associates Inc. (Gore) announced the first patient implant of the GORE TAG Conformable Thoracic Stent Graft with ACTIVE CONTROL System after receiving CE Mark last month. The first implant was performed by Prof. Dr. med. Giovanni Torsello and Dr. med. Martin Austermann at St. Franziskus Hospital in Munster, Germany.
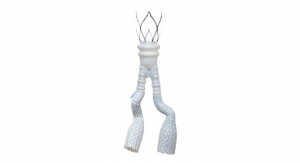
The thoracic endovascular aortic repair (TEVAR) device is the first to feature a new delivery system that provides the physician with controlled, staged deployment. The system optimizes accuracy, angulation, and apposition to treat etiologies of the descending thoracic aorta including aneurysms, transections, and acute and chronic Type B dissections. The new device will be formally launched in European regions later this year.
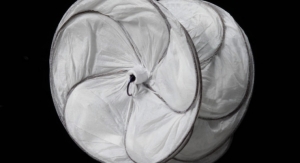
The GORE ACTIVE CONTROL System enhances the exceptional conformability of the stent graft; facilitating the optimized wall apposition that the Conformable GORE TAG Device is renowned for even in complex anatomies, such as acute aortic angles. The staged deployment feature enables the physician to refine positioning and angulate the stent graft within the body to achieve optimal placement prior to full-diameter expansion. The angulation control capability gives physicians the option to angulate the device to achieve orthogonal placement to the aortic blood flow lumen and maximize conformability and seal. These features enable physicians to more confidently perform endovascular treatment even in anatomies where tortuous vessels might historically have suggested open surgery.
“The enhanced control capabilities this new delivery system allows could reduce many common complications that can occur if the stent graft is not placed correctly during the TEVAR procedure,” commented Torsello. “Secondary interventions can be traumatic for patients and costly to the provider. The staged deployment feature of the GORE ACTIVE CONTROL System provides a level of precision that has never existed in TEVAR. This level of control in combination with the long-term benefits of the stent graft is a significant advancement for the field.”
The new product offering features the same time-tested stent graft as the Conformable GORE TAG Device, whose predictable outcomes have been established through its long-term freedom from reintervention (89 percent) and low serious endoleak (6 percent) and migration (0 percent) rates through five years.* The device is a unique combination of proprietary ePTFE graft material and a fully supported, nested, nitinol stent.
“Since Gore pioneered the first TEVAR device in Europe two decades ago, we have welcomed feedback from our physician partners to innovate and evolve our stent graft offerings for better long-term patient care,” said Eric Zacharias, vascular business leader at Gore. “The GORE TAG Device family has a legacy of trusted performance and durability, and we knew we could build on that by enhancing control during deployment which would help make TEVAR procedures more predictable for physicians. Physicians can now deploy our thoracic stent graft in the descending thoracic aorta with more operative ease, even in those patients with extremely angulated aortic arches, and meet the clinical and practical challenges of TEVAR with confidence. With this latest product iteration, Gore is continuing its unparalleled commitment to developing solutions that advance endovascular solutions for diseases of the aorta.”
The GORE TAG Conformable Stent Graft with ACTIVE CONTROL System is part of the family of endovascular products that treat aortic disease. The portfolio of products includes the GORE EXCLUDER AAA Endoprosthesis for the treatment of abdominal aortic aneurysms (AAA), as well as the GORE EXCLUDER Iliac Branch Endoprosthesis (IBE), the first U.S. Food and Drug Administration-approved off-the-shelf device indicated for the endovascular treatment of common iliac artery aneurysms or aortoiliac aneurysms. For potential additions to Gore’s branched portfolio, studies are ongoing for the GORE EXCLUDER Thoracoabdominal Branch Endoprosthesis (TAMBE), and enrollment continues in the GORE TAG Thoracic Branch Endoprosthesis (TBE) Pivotal Study to assess safety and effectiveness in treating lesions of the aortic arch and descending thoracic aorta.
* TAG 08-03 clinical trial for treatment of aneurysms of the descending thoracic aorta.
Gore Medical Products Division engineers devices that treat a range of cardiovascular and other health conditions. With more than 40 million medical devices implanted over the course of more than 40 years, Gore builds on its legacy of improving patient outcomes through research, education and quality initiatives.
W. L. Gore & Associates is a global materials science company dedicated to transforming industries and improving lives. Founded in 1958, Gore has built a reputation for solving complex technical challenges in the most demanding environments—from revolutionizing the outerwear industry with GORE-TEX fabric to creating medical devices that improve and save lives to enabling new levels of performance in the aerospace, pharmaceutical and mobile electronics markets, among other industries. Headquartered in Newark, Del., Gore employs approximately 10,000 associates and generates annual revenues that exceed $3 billion.
The thoracic endovascular aortic repair (TEVAR) device is the first to feature a new delivery system that provides the physician with controlled, staged deployment. The system optimizes accuracy, angulation, and apposition to treat etiologies of the descending thoracic aorta including aneurysms, transections, and acute and chronic Type B dissections. The new device will be formally launched in European regions later this year.
The GORE ACTIVE CONTROL System enhances the exceptional conformability of the stent graft; facilitating the optimized wall apposition that the Conformable GORE TAG Device is renowned for even in complex anatomies, such as acute aortic angles. The staged deployment feature enables the physician to refine positioning and angulate the stent graft within the body to achieve optimal placement prior to full-diameter expansion. The angulation control capability gives physicians the option to angulate the device to achieve orthogonal placement to the aortic blood flow lumen and maximize conformability and seal. These features enable physicians to more confidently perform endovascular treatment even in anatomies where tortuous vessels might historically have suggested open surgery.
“The enhanced control capabilities this new delivery system allows could reduce many common complications that can occur if the stent graft is not placed correctly during the TEVAR procedure,” commented Torsello. “Secondary interventions can be traumatic for patients and costly to the provider. The staged deployment feature of the GORE ACTIVE CONTROL System provides a level of precision that has never existed in TEVAR. This level of control in combination with the long-term benefits of the stent graft is a significant advancement for the field.”
The new product offering features the same time-tested stent graft as the Conformable GORE TAG Device, whose predictable outcomes have been established through its long-term freedom from reintervention (89 percent) and low serious endoleak (6 percent) and migration (0 percent) rates through five years.* The device is a unique combination of proprietary ePTFE graft material and a fully supported, nested, nitinol stent.
“Since Gore pioneered the first TEVAR device in Europe two decades ago, we have welcomed feedback from our physician partners to innovate and evolve our stent graft offerings for better long-term patient care,” said Eric Zacharias, vascular business leader at Gore. “The GORE TAG Device family has a legacy of trusted performance and durability, and we knew we could build on that by enhancing control during deployment which would help make TEVAR procedures more predictable for physicians. Physicians can now deploy our thoracic stent graft in the descending thoracic aorta with more operative ease, even in those patients with extremely angulated aortic arches, and meet the clinical and practical challenges of TEVAR with confidence. With this latest product iteration, Gore is continuing its unparalleled commitment to developing solutions that advance endovascular solutions for diseases of the aorta.”
The GORE TAG Conformable Stent Graft with ACTIVE CONTROL System is part of the family of endovascular products that treat aortic disease. The portfolio of products includes the GORE EXCLUDER AAA Endoprosthesis for the treatment of abdominal aortic aneurysms (AAA), as well as the GORE EXCLUDER Iliac Branch Endoprosthesis (IBE), the first U.S. Food and Drug Administration-approved off-the-shelf device indicated for the endovascular treatment of common iliac artery aneurysms or aortoiliac aneurysms. For potential additions to Gore’s branched portfolio, studies are ongoing for the GORE EXCLUDER Thoracoabdominal Branch Endoprosthesis (TAMBE), and enrollment continues in the GORE TAG Thoracic Branch Endoprosthesis (TBE) Pivotal Study to assess safety and effectiveness in treating lesions of the aortic arch and descending thoracic aorta.
* TAG 08-03 clinical trial for treatment of aneurysms of the descending thoracic aorta.
Gore Medical Products Division engineers devices that treat a range of cardiovascular and other health conditions. With more than 40 million medical devices implanted over the course of more than 40 years, Gore builds on its legacy of improving patient outcomes through research, education and quality initiatives.
W. L. Gore & Associates is a global materials science company dedicated to transforming industries and improving lives. Founded in 1958, Gore has built a reputation for solving complex technical challenges in the most demanding environments—from revolutionizing the outerwear industry with GORE-TEX fabric to creating medical devices that improve and save lives to enabling new levels of performance in the aerospace, pharmaceutical and mobile electronics markets, among other industries. Headquartered in Newark, Del., Gore employs approximately 10,000 associates and generates annual revenues that exceed $3 billion.