Business Wire07.24.17
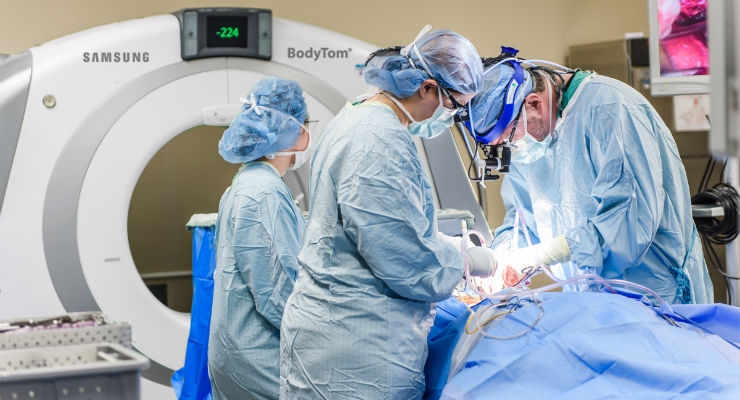
Samsung’s BodyTom, the world’s first portable, full-body, 32-slice CT (computed tomography) scanner, just got even better. The BodyTom Elite is the newly upgraded BodyTom, which received FDA 510(k) clearance on June 14, 2017. Incorporating a fresh new visual design, upgraded software, hardware and workstation the BodyTom Elite will make its mark as the go-to premium advanced full-body mobile CT scanner.
“We reengineered the BodyTom Elite so that it more seamlessly integrates into any OR, Neurosurgery, Radiation Oncology, or Radiology Suite, and provides a more accurate, sharper image,” said CEO Philip Sullivan.
In the world of advanced imaging, millimeters can be the difference when it comes to delivering an accurate diagnosis or treatment. With this in mind, Samsung’s team of R&D engineers spent the past year designing and improving hardware solutions, such as:
Significant improvements were also made to the workstation, including:
The BodyTom Elite software offers a slew of features, including:
“We run a very busy department and depend on our equipment to deliver the highest quality results possible with ease to the patient. The Samsung BodyTom Elite does all the above and more allowing for safe, precise, and effective treatment. The state-of-the-art BodyTom Elite features advanced technology including intuitive ergonomics, whisper-quiet portability, and exceptional image quality only to improve our department’s efficiency and patient comfort,” stated Michael Woodruff, director of medical imaging at John Muir Health.
BodyTom Elite is XR-29 (MITA Smart Dose) compliant and as such includes four key features of CT equipment that enable optimization or management of radiation dose delivery—dose structured reporting, CT dose check, AEC and pediatric and adult reference protocols. The BodyTom Elite remains a self-shielded, multi-departmental imaging solution capable of transforming any room in the hospital into an advanced imaging suite. The system can accommodate patients of all sizes, and its combination of rapid scan time, flexible settings, and immediate image viewing makes the BodyTom Elite a valuable tool to any facility needing versatile real-time portable imaging.
“From top to bottom, the BodyTom Elite is designed to empower our customers to capture the absolute best imaging information from their patients,” said Sullivan.
“We reengineered the BodyTom Elite so that it more seamlessly integrates into any OR, Neurosurgery, Radiation Oncology, or Radiology Suite, and provides a more accurate, sharper image,” said CEO Philip Sullivan.
In the world of advanced imaging, millimeters can be the difference when it comes to delivering an accurate diagnosis or treatment. With this in mind, Samsung’s team of R&D engineers spent the past year designing and improving hardware solutions, such as:
- Linear and step calibration delivering navigation accuracy for axial and helical scanning.
- Tilt sensor indicating if there are any significant shifts on the floor.
- Step Correction feature, which makes automatic adjustments if there are slight movements.
- New, quieter lift system reducing setup time, aligns with tables easier and allows for faster transition to scanning.
- New translate system, which pushes the centipede belts to the floor, reducing vibration and thereby improving image accuracy.
Significant improvements were also made to the workstation, including:
- Battery backup, meaning it can be transported without shutting it down, saving time.
- 2-way patient communication system allowing important information to be conveyed, easing patients as they undergo testing.
- More processing speed including 32 gigs of RAM and an upgraded processor.
- More export options including the ability to export datasets to DICOM format and view on PACs.
The BodyTom Elite software offers a slew of features, including:
- Low dose lung cancer screening under 3mGy to detect abnormalities.
- Contrast capability, which allows use of CT angiography and perfusion imaging utilizing bolus tracking and manual start.
- Metal Artifact Reduction (MAR) improving metal implants and trauma cases.
- Intuitive customization, such as the ability to change the slice width and gap, oblique the dataset and render in Maximum Intensity Projection, Minimum Intensity Projection, and Mean Value Projection.
- A brand-new noise-reduction algorithm, which will vastly improve image quality.
- 30 new custom kernels enabling a technologist to choose the sharpness, tissue contrast, and smoothing for each scan type.
- Automatic exposure control, which minimizes the amount of radiation by factoring the thickness and density of a patient.
- Wireless access point providing better security and stability of wireless communication by creating a standalone wireless network for the scanner compared to the existing Ad-Hoc environment.
“We run a very busy department and depend on our equipment to deliver the highest quality results possible with ease to the patient. The Samsung BodyTom Elite does all the above and more allowing for safe, precise, and effective treatment. The state-of-the-art BodyTom Elite features advanced technology including intuitive ergonomics, whisper-quiet portability, and exceptional image quality only to improve our department’s efficiency and patient comfort,” stated Michael Woodruff, director of medical imaging at John Muir Health.
BodyTom Elite is XR-29 (MITA Smart Dose) compliant and as such includes four key features of CT equipment that enable optimization or management of radiation dose delivery—dose structured reporting, CT dose check, AEC and pediatric and adult reference protocols. The BodyTom Elite remains a self-shielded, multi-departmental imaging solution capable of transforming any room in the hospital into an advanced imaging suite. The system can accommodate patients of all sizes, and its combination of rapid scan time, flexible settings, and immediate image viewing makes the BodyTom Elite a valuable tool to any facility needing versatile real-time portable imaging.
“From top to bottom, the BodyTom Elite is designed to empower our customers to capture the absolute best imaging information from their patients,” said Sullivan.