PR Newswire05.09.17
BIOTRONIK announced FDA approval and the launch of Sentus ProMRI, the thinnest quadripolar left ventricular lead available in the United States. This introduction completes BIOTRONIK's second-generation ProMRI lead portfolio, which also includes Solia ProMRI and Plexa ProMRI.
Sentus ProMRI is approved for use with heart failure devices based on data collected during the QP ExCELs study. The study, which included 53 sites in the United States, demonstrated Sentus ProMRI QP's best in class performance. Key data points include:
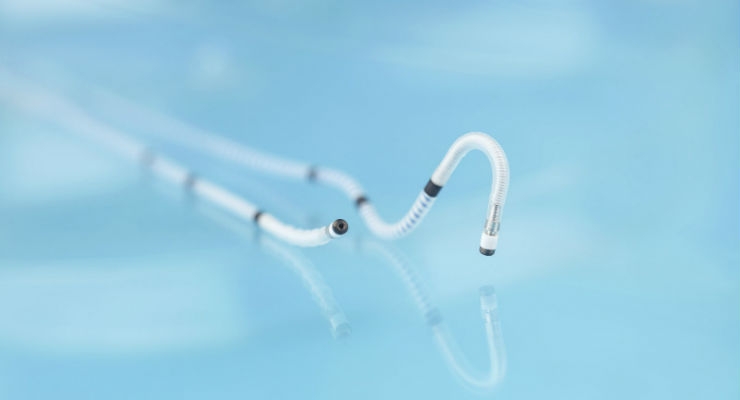
As more patients are living longer with cardiac conditions, BIOTRONIK continues to invest in research and development to bring solutions to market that improve quality of life for patients. Sentus ProMRI is a quadripolar coronary sinus left ventricular (LV) lead with an isodiametric design that marries co-radial wire insulation technology and polyurethane coating for maximum flexibility and reduced friction. The lead's four electrodes feature BIOTRONIK's proven fractal iridium coating to maximize performance.
"With the array of leads available today, every patient should be implanted with an MR conditional device," said Dr. Venkat R. Iyer, electrophysiologist at Bayview Physicians Group in Norfolk, Virginia. "Our patients deserve nothing less than the best possible care. With MR conditional solutions, we can help ensure this continues throughout the patient care journey."
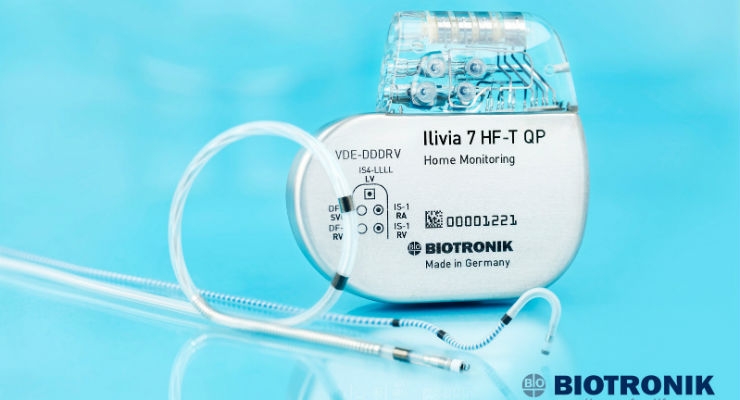
Sentus ProMRI completes the ProMRI quadripolar system when used with BIOTRONIK's Inventra HF-T QP cardiac resynchronization therapy defibrillator (CRT-D), making it the only MR conditional heart failure solution available in the United States that delivers ultra-high energy (42J) therapy on the first and every shock. This system offers Closed Loop Stimulation (CLS)—BIOTRONIK's unique physiologic rate-adaptive algorithm that automatically optimizes to each patient's needs based on real-time metabolic demand. It also features BIOTRONIK Home Monitoring, which has shown a 50% reduction in mortality1 in patients with heart failure, as proven in the IN-TIME study.
"After more than 50 years with zero lead recalls, we continue to invest in solutions that positively impact patient lives," said Marlou Janssen, president at BIOTRONIK Inc. "By equipping physicians with the most impactful cardiovascular solutions to confidently treat patients, we reinforce BIOTRONIK's dedication to advancing innovation that matters to health systems, physicians and patients."
The company's MultiPole Pacing (MPP) technology was also approved, providing physicians with additional treatment options for heart failure patients who have been non-responsive to cardiac resynchronization therapy (CRT).2 MPP will be available on new BIOTRONIK CRT defibrillator (CRT-D) systems for patients with heart failure.
Nearly 40 percent of heart failure patients are initially non-responsive to CRT.3 BIOTRONIK's innovative MPP technology addresses this challenge by enabling the left ventricle to be paced twice per cardiac cycle. Uniquely, these paces can be either sequential or simultaneous, allowing for greater customization of therapy to meet specific patient needs. BIOTRONIK CRT-D systems include MPP and feature ProMRI technology, providing patients with access to critical diagnostic imaging scans as needed. These devices are also equipped with MRI AutoDetect, a dedicated sensor that detects the MRI environment, converts the patient's device to MRI mode, and then automatically returns to its permanent program when the scan is complete.
With the availability of MPP, BIOTRONIK now offers the most comprehensive MR conditional CRT portfolio available today to ensure physicians have access to the best possible cardiac rhythm management solution.
"MultiPole Pacing is an important technology that allows physicians to tailor cardiac resynchronization therapy to each patient," said Dr. Gery Tomassoni, Baptist Health, Lexington, Kentucky. "Heart failure is a complex condition and physicians are routinely challenged to find the ideal treatment for unique disease presentations. Adding MPP technology to other key BIOTRONIK features creates more options for physicians to meet evolving patient needs."
"When we develop technologies and evolve our devices, we think first and foremost about patients. Patients deserve the best care possible and innovation that truly enhances everyday life," said Janssen. "Patients with heart failure are the most complex to treat, and they often have additional comorbidities that are likely to change and worsen over time. BIOTRONIK rises to this challenge by continuing to innovate, and offering impactful, customizable solutions to physicians that meet the ever-changing landscape of patients' needs."
BIOTRONIK's 360o CRT technologies are designed to better equip physicians to meet the therapy needs of heart failure patients. These technologies include:
References
1Hindricks G, et al. The Lancet. 2014, 384 (9943).
2Outcomes from use of BIOTRONIK MultiPole Pacing feature may be different than results obtained during St. Jude Medical MultiPoint Pacing (MPP) IDE study of their similar feature. The clinical effectiveness of this feature has not been established. BIOTRONIK is conducting an FDA-required post approval study to demonstrate that the MultiPole Pacing feature is effective by converting a percentage of CRT non-responders to responders.
3Mullens W et al. JACC. 2009, 53 (9).
4Hindricks G, et al. The Lancet. 2014, 384 (9943).
Sentus ProMRI is approved for use with heart failure devices based on data collected during the QP ExCELs study. The study, which included 53 sites in the United States, demonstrated Sentus ProMRI QP's best in class performance. Key data points include:
- 97.1 percent complication-free rate at six months
- 1.43 percent lead dislodgement rate
- 93.4 percent of subjects achieved permanent pacing vector pacing threshold lower than 2.5 V at three months
As more patients are living longer with cardiac conditions, BIOTRONIK continues to invest in research and development to bring solutions to market that improve quality of life for patients. Sentus ProMRI is a quadripolar coronary sinus left ventricular (LV) lead with an isodiametric design that marries co-radial wire insulation technology and polyurethane coating for maximum flexibility and reduced friction. The lead's four electrodes feature BIOTRONIK's proven fractal iridium coating to maximize performance.
"With the array of leads available today, every patient should be implanted with an MR conditional device," said Dr. Venkat R. Iyer, electrophysiologist at Bayview Physicians Group in Norfolk, Virginia. "Our patients deserve nothing less than the best possible care. With MR conditional solutions, we can help ensure this continues throughout the patient care journey."
Sentus ProMRI completes the ProMRI quadripolar system when used with BIOTRONIK's Inventra HF-T QP cardiac resynchronization therapy defibrillator (CRT-D), making it the only MR conditional heart failure solution available in the United States that delivers ultra-high energy (42J) therapy on the first and every shock. This system offers Closed Loop Stimulation (CLS)—BIOTRONIK's unique physiologic rate-adaptive algorithm that automatically optimizes to each patient's needs based on real-time metabolic demand. It also features BIOTRONIK Home Monitoring, which has shown a 50% reduction in mortality1 in patients with heart failure, as proven in the IN-TIME study.
"After more than 50 years with zero lead recalls, we continue to invest in solutions that positively impact patient lives," said Marlou Janssen, president at BIOTRONIK Inc. "By equipping physicians with the most impactful cardiovascular solutions to confidently treat patients, we reinforce BIOTRONIK's dedication to advancing innovation that matters to health systems, physicians and patients."
The company's MultiPole Pacing (MPP) technology was also approved, providing physicians with additional treatment options for heart failure patients who have been non-responsive to cardiac resynchronization therapy (CRT).2 MPP will be available on new BIOTRONIK CRT defibrillator (CRT-D) systems for patients with heart failure.
Nearly 40 percent of heart failure patients are initially non-responsive to CRT.3 BIOTRONIK's innovative MPP technology addresses this challenge by enabling the left ventricle to be paced twice per cardiac cycle. Uniquely, these paces can be either sequential or simultaneous, allowing for greater customization of therapy to meet specific patient needs. BIOTRONIK CRT-D systems include MPP and feature ProMRI technology, providing patients with access to critical diagnostic imaging scans as needed. These devices are also equipped with MRI AutoDetect, a dedicated sensor that detects the MRI environment, converts the patient's device to MRI mode, and then automatically returns to its permanent program when the scan is complete.
With the availability of MPP, BIOTRONIK now offers the most comprehensive MR conditional CRT portfolio available today to ensure physicians have access to the best possible cardiac rhythm management solution.
"MultiPole Pacing is an important technology that allows physicians to tailor cardiac resynchronization therapy to each patient," said Dr. Gery Tomassoni, Baptist Health, Lexington, Kentucky. "Heart failure is a complex condition and physicians are routinely challenged to find the ideal treatment for unique disease presentations. Adding MPP technology to other key BIOTRONIK features creates more options for physicians to meet evolving patient needs."
"When we develop technologies and evolve our devices, we think first and foremost about patients. Patients deserve the best care possible and innovation that truly enhances everyday life," said Janssen. "Patients with heart failure are the most complex to treat, and they often have additional comorbidities that are likely to change and worsen over time. BIOTRONIK rises to this challenge by continuing to innovate, and offering impactful, customizable solutions to physicians that meet the ever-changing landscape of patients' needs."
BIOTRONIK's 360o CRT technologies are designed to better equip physicians to meet the therapy needs of heart failure patients. These technologies include:
- MultiPole Pacing—enables left ventricle to be paced sequentially or simultaneously
- MRI AutoDetect— shortens window of reduced therapy and eliminates the need for post-MRI reprogramming
- Closed Loop Stimulation—equips the device to respond appropriately to physiologic demands and acute mental stress
- Ultra-High Energy CRT defibrillator—provides up to 42J of energy to meet patient needs on first and every shock
- CRT-DX system—allows full CRT-D therapy with only two leads
- BIOTRONIK Home Monitoring—automatically transmits device data daily; proven to reduce mortality in patients with heart failure by 50%4
References
1Hindricks G, et al. The Lancet. 2014, 384 (9943).
2Outcomes from use of BIOTRONIK MultiPole Pacing feature may be different than results obtained during St. Jude Medical MultiPoint Pacing (MPP) IDE study of their similar feature. The clinical effectiveness of this feature has not been established. BIOTRONIK is conducting an FDA-required post approval study to demonstrate that the MultiPole Pacing feature is effective by converting a percentage of CRT non-responders to responders.
3Mullens W et al. JACC. 2009, 53 (9).
4Hindricks G, et al. The Lancet. 2014, 384 (9943).