ASTM International03.21.17
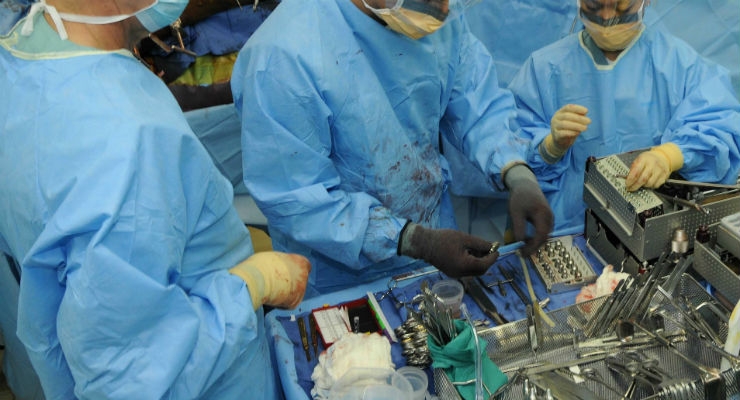
A new ASTM International guide will help the medical industry create and select artificial materials used to test the cleanliness of reusable medical devices.
The guide provides formulas for preparing artificial “test soils” that resemble blood, feces, and other biological matter that simulates contamination of the devices. Prior to this standard, there were few formulations available, according to ASTM International member Stephen Spiegelberg of Cambridge Polymer Group.
“This standard will be primarily used by medical-device manufacturers that make reusable devices, such as surgical instruments, pumps, and endoscopes,” said Spiegelberg. “Obviously, strong cleaning protocols are crucial when reprocessing medical devices between each use.”
In addition to manufacturers, independent testing laboratories might use the standard to create test soils and to develop and validate instructions for cleaning. Regulatory agencies that review submissions for reusable devices might also use the standard to compare the efficacy of various cleaning systems that use the same test soil(s).
The new standard (soon to be published as F3208, Guide for Selecting Test Soils for Validation of Cleaning Methods for Reusable Medical Devices) was developed by ASTM’s committee on medical and surgical devices.
In addition to formulations, the new standard provides procedures for selecting the appropriate test soil for different kinds of devices.
The guide provides formulas for preparing artificial “test soils” that resemble blood, feces, and other biological matter that simulates contamination of the devices. Prior to this standard, there were few formulations available, according to ASTM International member Stephen Spiegelberg of Cambridge Polymer Group.
“This standard will be primarily used by medical-device manufacturers that make reusable devices, such as surgical instruments, pumps, and endoscopes,” said Spiegelberg. “Obviously, strong cleaning protocols are crucial when reprocessing medical devices between each use.”
In addition to manufacturers, independent testing laboratories might use the standard to create test soils and to develop and validate instructions for cleaning. Regulatory agencies that review submissions for reusable devices might also use the standard to compare the efficacy of various cleaning systems that use the same test soil(s).
The new standard (soon to be published as F3208, Guide for Selecting Test Soils for Validation of Cleaning Methods for Reusable Medical Devices) was developed by ASTM’s committee on medical and surgical devices.
In addition to formulations, the new standard provides procedures for selecting the appropriate test soil for different kinds of devices.