Medtronic plc03.02.17
Medtronic plc has announced an economic analysis of five-year data showing that patients with mild heart failure who get cardiac resynchronization therapy (CRT) devices early in their treatment live longer and that implanting these devices is cost-effective, compared to optimal medical therapy. Results from the REVERSE (REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction) trial were published in the Journal of the American College of Cardiology: Heart Failure.
CRT is an established treatment for indicated patients with heart failure that has been demonstrated to improve survival and quality of life, and reduce hospitalizations. However, despite significant clinical evidence and guideline recommendations in support of CRT, studies have shown the therapy to be underutilized in eligible patients1.
REVERSE is the largest study to assess the long-term clinical impact and survival benefit of devices combining CRT with a defibrillator compared with CRT pacemakers. It is also the first study to show the cost-effectiveness of CRT when implanted earlier in the disease state.
“These new data expand upon the current evidence and guidelines for the treatment of heart failure, by showing that CRT in patients with mildly symptomatic heart failure is beneficial, both from a clinical perspective, as well as from a financial perspective,” said Michael R. Gold, M.D., Ph.D., chief of cardiology, Michael E Assey Professor of Medicine at the Medical University of South Carolina. “REVERSE confirms that implanting CRT earlier slows the progression of heart failure, reduces heart failure-related hospitalizations and deaths, and prolongs life, all while being very cost-effective.”
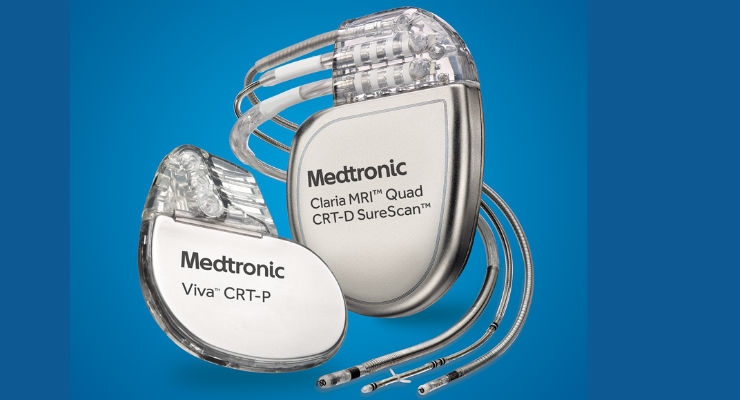
REVERSE was a prospective, randomized, double-blind study of 610 patients with mild heart failure (those designated New York Heart Association Class I/II) from North America and Europe. All patients were implanted with a CRT-pacemaker (CRT-P) or -defibrillator (CRT-D) and randomly assigned (2:1) to either CRT-ON or CRT-OFF.
Previously published REVERSE findings showed a trend that CRT-ON increased survival by nearly 23 percent (52.5 percent vs. 29.7 percent, p=0.21), leading to an expected survival rate of 9.76 years for CRT-ON versus 7.5 years for CRT-OFF2.
Based on these findings, the new analysis shows—for the first time under the Medicare setting—that CRT is a cost-effective option for patients with mild heart failure: CRT-ON yields an incremental cost effectiveness ratio (ICER) of $8,840 per Quality-Adjusted Life Year (QALY) gained over a patient’s lifetime, compared to CRT-OFF. (ICER is a statistic that summarizes the cost-effectiveness of a healthcare intervention, and QALY is a measure of the quantity and quality of life.)
Additionally, REVERSE has shown that CRT-D provides a significant improvement in survival—2.77 additional years of life—compared to CRT-P devices2. This benefit results in a first-of-its-kind finding that CRT-D is a cost-effective alternative to CRT-P, yielding an ICER of $43,678/QALY gained over the patient’s lifetime, lower than the benchmark for therapy cost effectiveness of other serious chronic conditions that cost at least $50,000 per QALY gained. Thus, while CRT-D costs more than CRT-P, the added 2.77 years of life justify the additional cost over a patient’s lifetime2.
Finally, these analyses show CRT delays disease progression, which means that initially implanting a CRT-D is essentially cost-neutral compared to implanting an ICD and implanting a CRT-D later, when the disease worsens. With early CRT-D implantation slowing disease progress and increasing survival, and without any discounting of future benefits and cost applied, early CRT-D led to 1.24 years of additional survival, resulting in an ICER of $1,829 per year of life gained.
“While CRT has long been established as a therapy that significantly improves outcomes for patients with heart failure, it is consistently underutilized,” said David Steinhaus, M.D., vice president and general manager of the Heart Failure business, and medical director for the Cardiac Rhythm and Heart Failure division of Medtronic. “Not only does REVERSE demonstrate the clinical benefit of CRT, it also quantifies the economic value of CRT, providing hospital systems with valuable information to help make informed decisions about CRT as a treatment option and the optimal timing of CRT for patients with heart failure. Ultimately, the goal is to increase value by improving patient outcomes and optimizing costs.”
In collaboration with leading clinicians, researchers and scientists worldwide, Medtronic offers the broadest range of innovative medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias. The company strives to offer products and services of the highest quality that deliver clinical and economic value to healthcare consumers and providers around the world.
1 Fonarow GC, Yancy CW, Albert NM, et al. Improving the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting: (IMPROVE HF). Circulation. August 10, 2010;122(6):585-96.
2 Gold MR, Padhair A, Mealing S, et al. Long-Term Extrapolation of Clinical Benefits Among Patients With Mild Heart Failure Receiving Cardiac Resynchronization Therapy: Analysis of the 5-Year Follow-Up from the REVERSE Study. JACC Heart Fail. 2015 Sep;3(9):691-700.
CRT is an established treatment for indicated patients with heart failure that has been demonstrated to improve survival and quality of life, and reduce hospitalizations. However, despite significant clinical evidence and guideline recommendations in support of CRT, studies have shown the therapy to be underutilized in eligible patients1.
REVERSE is the largest study to assess the long-term clinical impact and survival benefit of devices combining CRT with a defibrillator compared with CRT pacemakers. It is also the first study to show the cost-effectiveness of CRT when implanted earlier in the disease state.
“These new data expand upon the current evidence and guidelines for the treatment of heart failure, by showing that CRT in patients with mildly symptomatic heart failure is beneficial, both from a clinical perspective, as well as from a financial perspective,” said Michael R. Gold, M.D., Ph.D., chief of cardiology, Michael E Assey Professor of Medicine at the Medical University of South Carolina. “REVERSE confirms that implanting CRT earlier slows the progression of heart failure, reduces heart failure-related hospitalizations and deaths, and prolongs life, all while being very cost-effective.”
REVERSE was a prospective, randomized, double-blind study of 610 patients with mild heart failure (those designated New York Heart Association Class I/II) from North America and Europe. All patients were implanted with a CRT-pacemaker (CRT-P) or -defibrillator (CRT-D) and randomly assigned (2:1) to either CRT-ON or CRT-OFF.
Previously published REVERSE findings showed a trend that CRT-ON increased survival by nearly 23 percent (52.5 percent vs. 29.7 percent, p=0.21), leading to an expected survival rate of 9.76 years for CRT-ON versus 7.5 years for CRT-OFF2.
Based on these findings, the new analysis shows—for the first time under the Medicare setting—that CRT is a cost-effective option for patients with mild heart failure: CRT-ON yields an incremental cost effectiveness ratio (ICER) of $8,840 per Quality-Adjusted Life Year (QALY) gained over a patient’s lifetime, compared to CRT-OFF. (ICER is a statistic that summarizes the cost-effectiveness of a healthcare intervention, and QALY is a measure of the quantity and quality of life.)
Additionally, REVERSE has shown that CRT-D provides a significant improvement in survival—2.77 additional years of life—compared to CRT-P devices2. This benefit results in a first-of-its-kind finding that CRT-D is a cost-effective alternative to CRT-P, yielding an ICER of $43,678/QALY gained over the patient’s lifetime, lower than the benchmark for therapy cost effectiveness of other serious chronic conditions that cost at least $50,000 per QALY gained. Thus, while CRT-D costs more than CRT-P, the added 2.77 years of life justify the additional cost over a patient’s lifetime2.
Finally, these analyses show CRT delays disease progression, which means that initially implanting a CRT-D is essentially cost-neutral compared to implanting an ICD and implanting a CRT-D later, when the disease worsens. With early CRT-D implantation slowing disease progress and increasing survival, and without any discounting of future benefits and cost applied, early CRT-D led to 1.24 years of additional survival, resulting in an ICER of $1,829 per year of life gained.
“While CRT has long been established as a therapy that significantly improves outcomes for patients with heart failure, it is consistently underutilized,” said David Steinhaus, M.D., vice president and general manager of the Heart Failure business, and medical director for the Cardiac Rhythm and Heart Failure division of Medtronic. “Not only does REVERSE demonstrate the clinical benefit of CRT, it also quantifies the economic value of CRT, providing hospital systems with valuable information to help make informed decisions about CRT as a treatment option and the optimal timing of CRT for patients with heart failure. Ultimately, the goal is to increase value by improving patient outcomes and optimizing costs.”
In collaboration with leading clinicians, researchers and scientists worldwide, Medtronic offers the broadest range of innovative medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias. The company strives to offer products and services of the highest quality that deliver clinical and economic value to healthcare consumers and providers around the world.
1 Fonarow GC, Yancy CW, Albert NM, et al. Improving the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting: (IMPROVE HF). Circulation. August 10, 2010;122(6):585-96.
2 Gold MR, Padhair A, Mealing S, et al. Long-Term Extrapolation of Clinical Benefits Among Patients With Mild Heart Failure Receiving Cardiac Resynchronization Therapy: Analysis of the 5-Year Follow-Up from the REVERSE Study. JACC Heart Fail. 2015 Sep;3(9):691-700.