Business Wire01.13.17
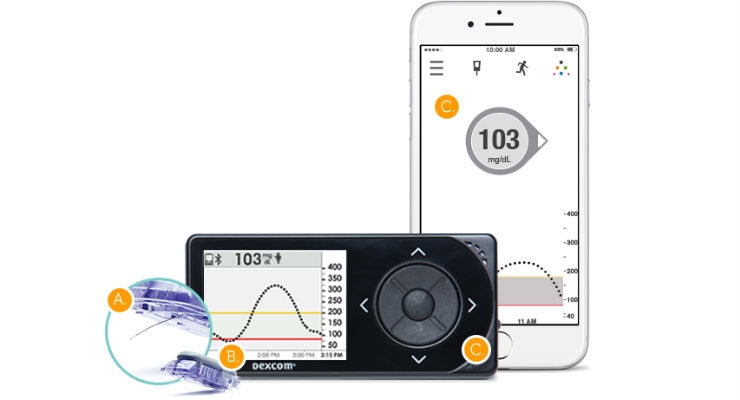
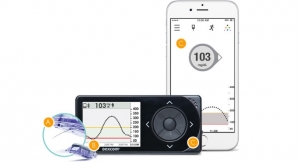
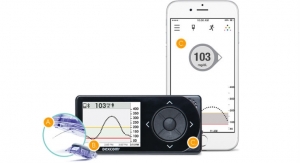
DexCom Inc., a provider of continuous glucose monitoring (CGM) for people with diabetes, is pleased to announce the determination of a benefit category and coverage for CGM by CMS. In order to be included in this category, the system must be defined as “therapeutic” CGM, meaning you can make treatment decisions using the device. Today, the Dexcom G5 Mobile is the only CGM system that falls within this classification. A link to the full CMS Ruling No. CMS-1682-R can be found at www.cms.gov/Regulations-and-Guidance/Guidance/Rulings/Downloads/CMS1682R.pdf.
“This landmark CMS Ruling will make available the most important technology in diabetes management to the Medicare population,” said Kevin Sayer, Dexcom President and Chief Executive Officer. “We are pleased with this important step forward and we look forward to working with Medicare on implementing coverage in the coming months to ensure beneficiaries have access to this life-saving device.”
“This landmark CMS Ruling will make available the most important technology in diabetes management to the Medicare population,” said Kevin Sayer, Dexcom President and Chief Executive Officer. “We are pleased with this important step forward and we look forward to working with Medicare on implementing coverage in the coming months to ensure beneficiaries have access to this life-saving device.”