Business Wire08.17.16
Teleflex Incorporated, a global provider of medical technologies for critical care and surgery, has officially launched its second-generation Percuvance Percutaneous Surgical System. The first clinical laparoscopic procedures in the United States using the system were performed at the Duke Center for Metabolic and Weight Loss Surgery in Durham, N.C.
Teleflex’s latest advance in percutaneous laparoscopy is more versatile and enables procedures to be even less invasive than its first-generation system while delivering the performance of 5 mm laparoscopic devices commonly used in minimally invasive surgeries (MIS) with less trauma.
The initial sleeve gastrectomy, Roux-en-Y gastric bypass, and laparoscopic gastric band removal procedures were performed by Dr. Dana D. Portenier and his team. Portenier is chief of general surgery at Duke Regional Hospital and the Division Chief at the Duke Center for Metabolic and Weight Loss Surgery.
“These are increasingly common but often challenging procedures,” said Portenier. “Our experiences with the new system are consistent with other clinical studies, which showed that its handling and functionality were similar to standard 5-mm laparoscopic instrumentation. Further research will determine whether the system’s smaller incisions could potentially demonstrate improvements in patient pain, cosmesis, and satisfaction.”
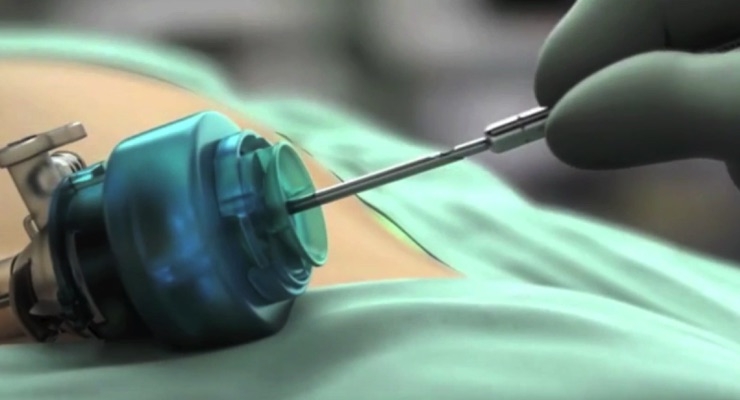
The second-generation, 2.9 mm Percuvance System features additional interchangeable 5-mm tool tips and a new quick-connect system to facilitate fast and secure tool-tip changes outside of the body. The system has the performance and versatility for use in common and advanced general laparoscopic procedures, including laparoscopic cholecystectomy, upper gastrointestinal, gastric, bariatric, colorectal and hernia. It enables surgeons to eliminate one or more additional trocars from their laparoscopic procedures versus the first-generation device while delivering the outcomes and performance they expect from their traditional laparoscopic devices.
The first-generation Percuvance System has been used by more than 150 surgeons in the United States, Europe, Middle East, and Africa since Teleflex implemented a limited release of the product in March 2015. The second-generation Percuvance device recently received U.S. Food and Drug Administration clearance and CE Mark approval. During the next month, a series of surgical procedures with the new Percuvance System will be conducted at other institutions in New York, Texas, Florida and California by surgeons with experience using the first-generation system.
“Our second-generation Percuvance System represents a significant advancement in the ongoing and perpetual evolution of minimally invasive surgery and is demonstrative of Teleflex’s commitment to innovation,” said John Tushar, president and general manager of Teleflex Surgical. “We are encouraged by surgeons’ enthusiastic response to our percutaneous surgical devices and are optimistic the new Percuvance System will become a new standard of care in laparoscopic surgery.”
Teleflex is ramping up its sales and marketing efforts to familiarize surgeons worldwide with the performance capabilities and benefits of the 2.9 mm Percuvance System.
Traditional laparoscopic surgery revolutionized surgical practices more than 30 years ago, and approximately 3 million laparoscopic procedures are performed each year in the United States.1 Since the early 2000s, new technologies and devices have been introduced with the goal of making these surgeries increasingly less invasive. None, however, has yet to become widely accepted in general practice for common laparoscopic procedures.
Percutaneous laparoscopy is a new category of laparoscopic surgery promoted by Teleflex to overcome the barriers that slowed widespread acceptance of other approaches. In developing the new Percuvance System, Teleflex listened to surgeons who said they wanted:
Teleflex’s Percuvance Percutaneous Surgical System is a family of instruments used to perform laparoscopic procedures. It includes components that introduce a variety of instrument configurations into the abdominal cavity and requires a smaller incision site than traditional laparoscopic surgery. It offers reusable handles and seven disposable 5 mm interchangeable tool tips, which include graspers, scissors, dissector, electrocautery tools, and a Weck Hem-o-lok Polymer Ligation Clip Applier. Unlike some other laparoscopic devices, the Percuvance System affords a percutaneous insertion into the patient without the use of an insertion trocar. The Percuvance Percutaneous Surgical System with 5 mm attachments is indicated for the means to penetrate soft tissue to access certain areas of the abdomen. The systems are used to grasp, manipulate, cut, cauterize, and deliver Hem-o-lok System ligating clips to soft tissue during laparoscopic surgery.
Watch the video below to learn more about the Percuvance Percutaneous Surgical System:
The Percuvance System complements another key product, the MiniLap Percutaneous Surgical System, in Teleflex’s portfolio of solutions for percutaneous laparoscopy. The MiniLap System provides delivery and function through fully integrated and disposable 2.3 mm and 2.4 mm devices. Like the Percuvance System, the MiniLap System does not require a trocar for initial access and makes minimally invasive procedures even less invasive for procedures where common retraction, manipulation, and monopolar energy are required.
Teleflex is a global provider of solutions in the fields of vascular and interventional access, surgical, anesthesia, cardiac care, urology, emergency medicine and respiratory care.
References:
1. Source: Millennium Research Group, Inc, RPUS30LA13 | November 2014, US MARKETS FOR LAPAROSCOPIC DEVICES 2015, p30, figure 4.
Teleflex’s latest advance in percutaneous laparoscopy is more versatile and enables procedures to be even less invasive than its first-generation system while delivering the performance of 5 mm laparoscopic devices commonly used in minimally invasive surgeries (MIS) with less trauma.
The initial sleeve gastrectomy, Roux-en-Y gastric bypass, and laparoscopic gastric band removal procedures were performed by Dr. Dana D. Portenier and his team. Portenier is chief of general surgery at Duke Regional Hospital and the Division Chief at the Duke Center for Metabolic and Weight Loss Surgery.
“These are increasingly common but often challenging procedures,” said Portenier. “Our experiences with the new system are consistent with other clinical studies, which showed that its handling and functionality were similar to standard 5-mm laparoscopic instrumentation. Further research will determine whether the system’s smaller incisions could potentially demonstrate improvements in patient pain, cosmesis, and satisfaction.”
The second-generation, 2.9 mm Percuvance System features additional interchangeable 5-mm tool tips and a new quick-connect system to facilitate fast and secure tool-tip changes outside of the body. The system has the performance and versatility for use in common and advanced general laparoscopic procedures, including laparoscopic cholecystectomy, upper gastrointestinal, gastric, bariatric, colorectal and hernia. It enables surgeons to eliminate one or more additional trocars from their laparoscopic procedures versus the first-generation device while delivering the outcomes and performance they expect from their traditional laparoscopic devices.
The first-generation Percuvance System has been used by more than 150 surgeons in the United States, Europe, Middle East, and Africa since Teleflex implemented a limited release of the product in March 2015. The second-generation Percuvance device recently received U.S. Food and Drug Administration clearance and CE Mark approval. During the next month, a series of surgical procedures with the new Percuvance System will be conducted at other institutions in New York, Texas, Florida and California by surgeons with experience using the first-generation system.
“Our second-generation Percuvance System represents a significant advancement in the ongoing and perpetual evolution of minimally invasive surgery and is demonstrative of Teleflex’s commitment to innovation,” said John Tushar, president and general manager of Teleflex Surgical. “We are encouraged by surgeons’ enthusiastic response to our percutaneous surgical devices and are optimistic the new Percuvance System will become a new standard of care in laparoscopic surgery.”
Teleflex is ramping up its sales and marketing efforts to familiarize surgeons worldwide with the performance capabilities and benefits of the 2.9 mm Percuvance System.
Traditional laparoscopic surgery revolutionized surgical practices more than 30 years ago, and approximately 3 million laparoscopic procedures are performed each year in the United States.1 Since the early 2000s, new technologies and devices have been introduced with the goal of making these surgeries increasingly less invasive. None, however, has yet to become widely accepted in general practice for common laparoscopic procedures.
Percutaneous laparoscopy is a new category of laparoscopic surgery promoted by Teleflex to overcome the barriers that slowed widespread acceptance of other approaches. In developing the new Percuvance System, Teleflex listened to surgeons who said they wanted:
- Even less invasive surgical practices that do not compromise performance
- Reduced trauma and scarring
- A device that didn’t require learning a new, complicated surgical technique.
Teleflex’s Percuvance Percutaneous Surgical System is a family of instruments used to perform laparoscopic procedures. It includes components that introduce a variety of instrument configurations into the abdominal cavity and requires a smaller incision site than traditional laparoscopic surgery. It offers reusable handles and seven disposable 5 mm interchangeable tool tips, which include graspers, scissors, dissector, electrocautery tools, and a Weck Hem-o-lok Polymer Ligation Clip Applier. Unlike some other laparoscopic devices, the Percuvance System affords a percutaneous insertion into the patient without the use of an insertion trocar. The Percuvance Percutaneous Surgical System with 5 mm attachments is indicated for the means to penetrate soft tissue to access certain areas of the abdomen. The systems are used to grasp, manipulate, cut, cauterize, and deliver Hem-o-lok System ligating clips to soft tissue during laparoscopic surgery.
Watch the video below to learn more about the Percuvance Percutaneous Surgical System:
The Percuvance System complements another key product, the MiniLap Percutaneous Surgical System, in Teleflex’s portfolio of solutions for percutaneous laparoscopy. The MiniLap System provides delivery and function through fully integrated and disposable 2.3 mm and 2.4 mm devices. Like the Percuvance System, the MiniLap System does not require a trocar for initial access and makes minimally invasive procedures even less invasive for procedures where common retraction, manipulation, and monopolar energy are required.
Teleflex is a global provider of solutions in the fields of vascular and interventional access, surgical, anesthesia, cardiac care, urology, emergency medicine and respiratory care.
References:
1. Source: Millennium Research Group, Inc, RPUS30LA13 | November 2014, US MARKETS FOR LAPAROSCOPIC DEVICES 2015, p30, figure 4.