Pulmonx Corporation12.11.15
Pulmonx Corporation's Chartis System and Zephyr Endobronchial Valve (EBV) is better at treating emphysema than standard methods, according to a clinical study published in the New England Journal of Medicine.
The Stelvio trial is the first independent, randomized, controlled trial of Zephyr EBV therapy using the Chartis System to identify patients most likely to benefit. It is also the first prospective trial to demonstrate that Zephyr EBV therapy can benefit a broad range of advanced stage emphysema patients, including those with heterogeneous disease, where emphysema is isolated to specific areas of the lungs, and homogeneous disease, where emphysema is distributed evenly throughout the lungs. The trial was performed independently of Pulmonx.
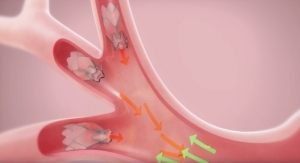
In the Stelvio trial, 68 patients were confirmed with the Chartis System to be likely responders to Zephyr EBV therapy, and randomized to either EBV therapy or medical management. In patients who received EBV therapy, tiny one-way valves were placed in the lungs to block airflow to diseased regions with the goal of improving breathing.
At six months, compared to the medical management group, the EBV group had statistically and clinically greater improvements in pulmonary function (FEV1 change percentage predicted, EBV vs. Control: 20.9 percent vs. 3.1 percent; p=0.001), exercise capacity (6-minute walk test, EBV vs. Control: +19.6 percent vss -3.6 percent; p=0.001) and quality of life (SGRQ score, EBV vs. Control: -21.8 vs. -7.6; p=0.001). Also, a significantly greater number of patients in the EBV group responded to treatment. At the end of six months, control patients that crossed over to receive EBV therapy gained benefits similar to the original EBV-treated group.
The Stelvio trial was led by Karin Klooster and Dirk-Jan Slebos of the Department of Pulmonary Diseases and the Groningen Research Institute for Asthma and COPD, University Medical Center Groningen, The Netherlands. The trial was funded by a Dutch government grant from ZonMW and the University Medical Center Groningen, The Netherlands.
“When we select patients with the Chartis System, endobronchial valve treatment provides improved lung function, exercise capacity, and quality of life,” said Karin Klooster, lead author of the publication. “When we identify the right patients for treatment, the improvements can be life-changing,“ said Principal Investigator Dr. Dirk-Jan Slebos.
“The Stelvio study provides independent confirmation that a broad range of patients with advanced emphysema have a high likelihood of achieving clinically meaningful benefits from Zephyr EBV therapy when a systematic approach is followed and careful patient selection is performed using the Chartis System,” said Pulmonx CEO Glen French.
The Zephyr EBV is a minimally-invasive treatment for severe emphysema that has been proven in over a decade of clinical experience to significantly improve the lung function, exercise tolerance and quality of life for patients receiving treatment. The Chartis System is utilized prior to the procedure to identify likely responders. In the procedure, tiny, one-way valves are placed in the lungs to block airflow to diseased regions to achieve lung volume reduction. As a result, the remaining healthy regions may function more efficiently, enabling better breathing and an improved quality of life for patients who can then perform more activities of daily life. Zephyr EBVs have been implanted globally in more than 10,000 patients.
Based in Redwood City, Calif., and Neuchâtel, Switzerland, Pulmonx is an interventional pulmonology company that develops lung disease treatment technologies.
Watch a video of the Zephyr EBV procedure below:
The Stelvio trial is the first independent, randomized, controlled trial of Zephyr EBV therapy using the Chartis System to identify patients most likely to benefit. It is also the first prospective trial to demonstrate that Zephyr EBV therapy can benefit a broad range of advanced stage emphysema patients, including those with heterogeneous disease, where emphysema is isolated to specific areas of the lungs, and homogeneous disease, where emphysema is distributed evenly throughout the lungs. The trial was performed independently of Pulmonx.
In the Stelvio trial, 68 patients were confirmed with the Chartis System to be likely responders to Zephyr EBV therapy, and randomized to either EBV therapy or medical management. In patients who received EBV therapy, tiny one-way valves were placed in the lungs to block airflow to diseased regions with the goal of improving breathing.
At six months, compared to the medical management group, the EBV group had statistically and clinically greater improvements in pulmonary function (FEV1 change percentage predicted, EBV vs. Control: 20.9 percent vs. 3.1 percent; p=0.001), exercise capacity (6-minute walk test, EBV vs. Control: +19.6 percent vss -3.6 percent; p=0.001) and quality of life (SGRQ score, EBV vs. Control: -21.8 vs. -7.6; p=0.001). Also, a significantly greater number of patients in the EBV group responded to treatment. At the end of six months, control patients that crossed over to receive EBV therapy gained benefits similar to the original EBV-treated group.
The Stelvio trial was led by Karin Klooster and Dirk-Jan Slebos of the Department of Pulmonary Diseases and the Groningen Research Institute for Asthma and COPD, University Medical Center Groningen, The Netherlands. The trial was funded by a Dutch government grant from ZonMW and the University Medical Center Groningen, The Netherlands.
“When we select patients with the Chartis System, endobronchial valve treatment provides improved lung function, exercise capacity, and quality of life,” said Karin Klooster, lead author of the publication. “When we identify the right patients for treatment, the improvements can be life-changing,“ said Principal Investigator Dr. Dirk-Jan Slebos.
“The Stelvio study provides independent confirmation that a broad range of patients with advanced emphysema have a high likelihood of achieving clinically meaningful benefits from Zephyr EBV therapy when a systematic approach is followed and careful patient selection is performed using the Chartis System,” said Pulmonx CEO Glen French.
The Zephyr EBV is a minimally-invasive treatment for severe emphysema that has been proven in over a decade of clinical experience to significantly improve the lung function, exercise tolerance and quality of life for patients receiving treatment. The Chartis System is utilized prior to the procedure to identify likely responders. In the procedure, tiny, one-way valves are placed in the lungs to block airflow to diseased regions to achieve lung volume reduction. As a result, the remaining healthy regions may function more efficiently, enabling better breathing and an improved quality of life for patients who can then perform more activities of daily life. Zephyr EBVs have been implanted globally in more than 10,000 patients.
Based in Redwood City, Calif., and Neuchâtel, Switzerland, Pulmonx is an interventional pulmonology company that develops lung disease treatment technologies.
Watch a video of the Zephyr EBV procedure below: