Validation of a molding process can be critical to the overall success of a medical device project. If there are missteps or unexpected delays due to poor preparation or a lack of clarity in identifying the important criteria of a part, the process could delay a product’s regulatory schedule and interrupt the anticipated time to market timeframe. As a result, it is important for medical device manufacturers and their supply partners to be aligned with expectations, communication, and the most critical aspects of a molded part.
While the medical device OEM may be familiar with what’s involved with a molding validation, they likely aren’t as knowledgeable with the protocols and, more importantly, the true critical factors they need to ensure are communicated to their supply chain partner. The sharing of best practices can help illustrate what the OEM can do to help make the process smooth.
With this in mind, Steve VanderKooi, the quality engineering manager at PTI Engineered Plastics—a Macomb, Mich.-based injection molder and manufacturer of plastic components and assemblies—shared some tips and considerations to ensure an optimal process. In this Q&A, VanderKooi highlighted common missteps, factors to consider, typical reasons for a delay, and suggestions for streamlining validation without sacrificing quality.
Sean Fenske: Since we’re discussing plastic injection molding validation, can you please outline the basic steps involved in this process?
Steve VanderKooi: I think most people are familiar with the installation qualification (IQ), operational qualification (OQ), and process performance qualification (PQ) steps of validation but what they may not be aware of is the most important step of all—process characterization.

This is an overview of the PTI injection molding validation process. It outlines the basic steps and shows our standardized work instructions and flow chart.
PTI characterizes new injection molding processes using scientific injection molding (SIM) principals. The foundation of a validated process is built from the SIM activity in conjunction with design of experiments (DOE) run data. PTI has developed a SIM workbook that is completed by our process engineers for each tool. Completion of our SIM workbook ensures a methodical approach to process development. The SIM study determines where machine settings need to be to create a short shot on the low end and parting line flash on the high end (tool blown open). Once the process extremes are known, the settings are tightened down further and this narrowed down range is used as the limits for the DOE activity. A full factorial DOE is performed, and the DOE data provides insight to the extent in which a part can be dimensionally changed by altering significant process parameters.
After DOE, we develop an OQ-PQ protocol which further reduces the process window. Parts are molded at the low, nominal, and high process settings during the OQ run and a summary report is generated compiling the data, manufacturing, and material records. Any discrepancies between the molded part and drawing requirements must be reconciled at this time via tooling or drawing changes. There should be no surprises going into the PQ runs.
Three PQ runs are molded using the nominal process settings identified in the OQ-PQ protocol. A PQ report is generated and sent to the customer for review and approval. Once the PQ report has been fully approved, parts are released and shipped to the customer.
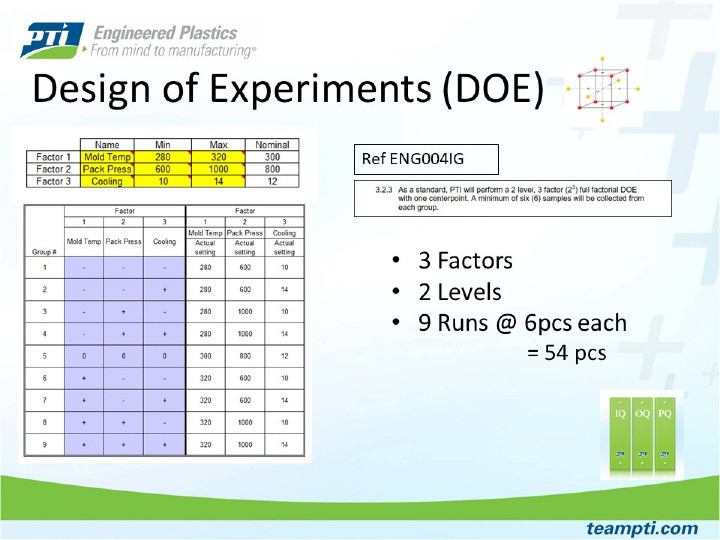
Shows the PTI standard setup for full factorial Design of Experiments.
Fenske: How long does this process typically take and what aspects are contributing most to that timeline?
VanderKooi: An injection molding process validation can take anywhere from 6 weeks to 8+ months depending on the scope of the validation, needs of the customer, and part design.
Given the large number of activities, departments, documentation, inspection, and manufacturing runs required to complete an injection molding validation, it is easy to see how some projects can take well over 8 months to complete.
A lack of a clear validation plan and defined part requirements can delay a project on the front end. Overly complicated dimensional and/or cosmetic requirements will often cause delays on the back end. If there is not a clear line of communication to the part designer, the decision whether to make a print change or tooling change can take weeks, if not months.
A quick review of our 10 most outstanding validation projects identified several common causes of project delays:
- Changes to project scope (e.g., design change, additional inspection criteria)
- Protocol/Report approval delays
- Excessive part requirements
Fenske: In your experience, what are some common misconceptions about the validation of plastic injection molding?
VanderKooi: Customers often feel there is something wrong with the injection molding process if a molded part does not meet all print specifications. There are many variables that can contribute to a part failing to meet print specification including part design, material, or tolerances exceeding tooling capabilities. We have performed countless dimensional verifications on injection molding tools and rarely find dimensions deviating from the tool design values.
Another common misconception is the idea that process settings have a large impact on part dimensions or that process settings can be modified to bring part dimensions into tolerance. This is rarely the case and changing the process to move one dimension will often result in moving other dimensions as well. To ensure success into production, injection molders should be running the best possible process to produce a consistent and cosmetically acceptable part. This “best” or nominal process is established using SIM principles and will be the center-point for the DOE study. If a critical dimension is out of specification, the best way to address it is to modify the tooling.
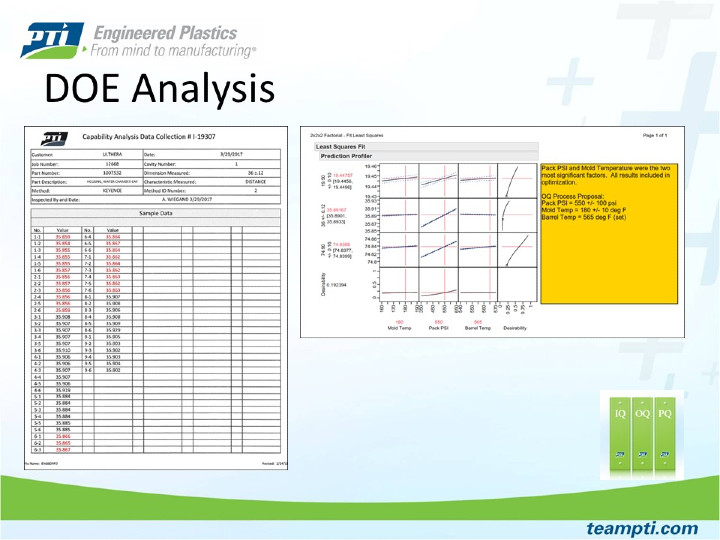
Shows the power of JMP software when analyzing DOE data with multiple responses (dimensions). This helps PTI customers fully understand how the process settings may or may not impact each part dimension. Once the analysis is complete, the software allows users to adjust process parameters up or down while showing the expected dimensional outputs.
Fenske: What advice would you give to manufacturers to help them avoid pitfalls and delays they may encounter in the validation process?
VanderKooi: A project without clearly defined part requirements/critical characteristics is destined for the slow lane. If the customer has not conveyed how critical dimensions will be inspected during validation/production, then this needs to become a number-one priority. I cannot count the number of validations that stalled while the teams “developed an inspection fixture” or “determined a repeatable way to measure”.
A few other factors to consider include:
- Adding more critical dimensions does not ensure a better quality part
- Tighter dimensional tolerances do not ensure a better tool or molding process
- Tools that do not run automatic (handloads) are not good candidates for validation
- Avoid validation of materials that must be hand mixed (i.e., colorant added)
Fenske: Since quality is paramount, how can you streamline validation without running the risk of sacrificing that quality?
VanderKooi: There are several ways PTI has streamlined our validation process.
Documentation: Our protocols are written to focus on dimensions critical to the process, meaning the dimensions most likely impacted by changes to the process. If we select the correct process monitoring dimensions, we can ensure all dimensions identified by the customer as critical will meet specified requirements.
Standardization: Through standardizing the validation process/protocols/reports, we ensure everyone at PTI is fully trained to execute validation activities.
Technology: We use technology to streamline our validation process. Our manufacturing records are electronic, which allows immediate access for review and inclusion in reports. PTI also implements automated inspection methods whenever possible, eliminating manual data collection, reducing inspection error, and reducing overall project timing.
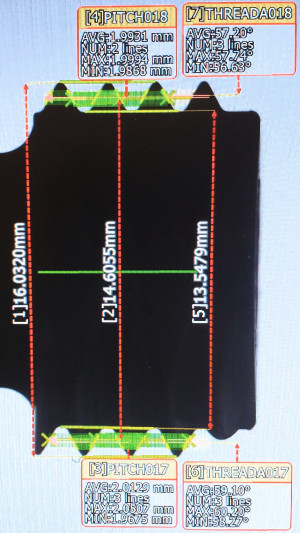
Shows use of automated inspection equipment to inspect threads on a part. All dimensional values shown were collected in less than three seconds with the push of a button. Automating inspection saves time and can greatly improve data integrity.
VanderKooi: It is imperative that inspection methods used during validation are repeatable, reproduceable, and will be the same inspection methods planned for ongoing production.
PTI has been at the forefront of automating inspection processes. We have invested heavily in automated inspection equipment (vision systems) and spent years training employees how to create and verify inspection programs. Given how easy it is to manipulate plastic parts with traditional inspection equipment (i.e., calipers), removing or reducing operator error from the measurement system ensures the integrity of data collected during validation into production.
Lastly, I would caution the use of excessive visual inspections to ensure part quality. If visual inspections must be relied upon, we recommend using industry standards such as those outlined by The Society of the Plastics Industry.
Fenske: Do you have any additional comments you’d like to share based on any of the topics we discussed or something you’d like to tell medical device manufacturers?
VanderKooi: Creating a compliant validation system with standardized procedures/forms/templates is not something that happens overnight. Training all necessary team members on all shifts to perform validations and complete the associated documentation takes time and dedication. Allowing customers to change or deviate from the established process adds to overall validation timing through additional training and execution mistakes.
Learn more about PTI Engineered Plastics Inc. >>>>>














