By Sean Fenske, Editor-in-Chief
Medical devices are becoming more advanced rapidly to address a multitude of needs within healthcare. As these products are being asked to do more, so too is the request being made of their components. As medtech becomes more sophisticated, the parts that make them up must also mature and advance.
One often overlooked, yet critical element of many types of medical devices is the tubing. When it comes to pushing the boundaries on enhancing the offering to serve as needed within today’s healthcare tools, suppliers are exceeding the limits previously achieved. As such, they are constantly innovating and developing new technique with which to stretch materials and machines to their limits to make an exceptional product.
Sharing insights on what his company is doing within the realm of tubing is Tim Steele, founder and CEO of Microspec Corporation. In the following Q&A, Steele offers his thoughts on a number of areas involving the development and production of medical tubing.
Sean Fenske: When you say “next gen medical tubing,” what are you really talking about?
A graphic display of a bump tube prototype.
Tim Steele: Next gen medical tubing means just that! For instance, MicroSpec has been a global leader in producing neonatal catheter tubing for over two decades. The first two tubes we introduced at Medtec in Stuttgart were somewhat slow to start, but the 1.9 Fr dual lumen bump tube soon became the global standard for neonatal catheters. We continue to manufacture these parts at high volumes today.
With neonatal treatment expanding, however, we at MicroSpec saw an opportunity to create a new catheter tube for a broader range of use. We designed and produced a three-lumen, 2.5 Fr catheter tube that we introduced to the industry at the 2023 MD&M show in Anaheim. This tube is an authentic 2nd generation of the 1.9 Fr dual lumen. The three-lumen bump tube has the attributes of the two-lumen bump tube, but the scope of its application goes beyond the two lumen and broadens it.
Fenske: What are you doing to remain able to develop and manufacture tubing that meets the ever-increasing demands of medical device manufacturers?
Steele: To stay abreast of the ever-expanding medical technologies that require extruded tubing and profiles, we listen and talk to the people conceiving the ideas that turn into the future medical technologies not yet in existence. Since the beginning of MicroSpec, the company has built a reputation on taking projects others would turn away. Of course, some of these projects did not succeed, but many do and that is how we grow from year to year. We have more than twenty-five high volume tubes/parts that we’ve been extruding for about 25 years or more and they all started out as a concept others had turned away as not worth doing or too complex to consider. We still follow this model of pushing the extrusion envelope and challenging ourselves to innovate to meet the needs of an ever-changing medical device landscape.
Fenske: Why are tubing demands increasing in complexity?
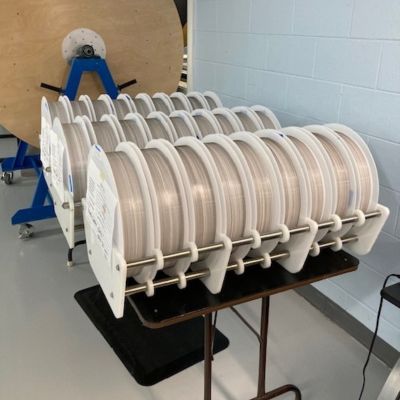
Handling and packaging very small, delicate tubing is technique-sensitive and challenging.
Steele: Tubing is getting increasingly complex because the tube is a reflection of the new medical technology being conceived. In general, the technology advance precedes the tubing advance as the new tube design/form is a response to the technology advance.
Fenske: In what types of medical applications is “next gen” tubing being used most often?
Steele: “Next gen” tubing is not confined to one or two applications as new development is happening everywhere in the medical device industry. I’ve already given an example of how neonatal tubing is morphing. It’s not just physical design that may define a tube as “next gen”; rather, the materials used for the tube change as well. The polyurethane family of raw materials are often reformulated with various additives to enhance mechanical and physical characteristics to improve performance. (Lubricious and anti-oxidant additives are the most common.)
Fenske: What are the major obstacles that need to be overcome when “pushing the boundaries” for next gen tubing? Is it an issue of equipment, materials, both, or something else entirely?
Very small, complex tubing is challenging to make extrusion tools for.
Steele: At MicroSpec, we encounter a variety of obstacles in developing “next gen” tubes and, in many cases, “first gen” tubing. Frequently, the new tube pushes the boundaries of what has been possible in the past and we are faced with a tube containing features we’ve never produced before. In this situation, the challenge typically has two sides to it. The first challenge is designing and fabricating an extrusion tool to extrude the new tube and the second challenge is the actual extrusion of the tube.
The challenge of machining the extrusion tools is often the most difficult and generally, the most common hurdle we must clear. Most often, the challenge involves making the machined tools small enough to produce complex tubing that is getting smaller and smaller as medical technology advances. Having a state-of-the-art machine shop is critical, which we have at MicroSpec. Some new tube designs have required us to use wire EDM with wires as small as 0.002 inches, which is a very slow and costly process. On the other hand, it allows us to achieve the intricacies in the tools that enable us to extrude the new tube.

Inspection is often complicated by unusual designs.
With every new design, we also face the challenges of developing a robust extrusion process and an effective inspection routine for the part. We know from experience developing the best techniques is critical to the success of the extruded part, as is having our inspection methods in alignment with the customer’s inspection methods. Measuring radii and ultra-thin walls relies upon technique as well, especially when the wall thicknesses are specified in microns. This has required us to measure the same wall several times, determine the average of all the readings, and then record.
For new tubes, we try to define all the challenges in the quoting process, but typically find something not anticipated. Another obstacle we run into is validation of the process and the part. Miniaturized parts are particularly challenging as the specified tolerances are so small the process uses almost the entire allowed tolerance and a 1.33 Cpk cannot be met. In cases like this, an acceptable Cpk needs to be agreed upon with the customer.
Fenske: Do you see areas for further optimization or ways to gain even greater benefits from tubing that’s not yet being utilized?
Steele: Making a new or next generation tube always leads to innovation. Making a new part the first time always shows us what we could have done better and even with parts that meet the customer’s specification the first time, we generally go back to re-work the tools and/or the extrusion process to optimize dimensions. It is our experience a part is not optimized before it has run at least three or four times, but we are always looking to improve the process.
Click here to find out more about MicroSpec >>>>>
Medical devices are becoming more advanced rapidly to address a multitude of needs within healthcare. As these products are being asked to do more, so too is the request being made of their components. As medtech becomes more sophisticated, the parts that make them up must also mature and advance.
One often overlooked, yet critical element of many types of medical devices is the tubing. When it comes to pushing the boundaries on enhancing the offering to serve as needed within today’s healthcare tools, suppliers are exceeding the limits previously achieved. As such, they are constantly innovating and developing new technique with which to stretch materials and machines to their limits to make an exceptional product.
Sharing insights on what his company is doing within the realm of tubing is Tim Steele, founder and CEO of Microspec Corporation. In the following Q&A, Steele offers his thoughts on a number of areas involving the development and production of medical tubing.
Sean Fenske: When you say “next gen medical tubing,” what are you really talking about?

A graphic display of a bump tube prototype.
With neonatal treatment expanding, however, we at MicroSpec saw an opportunity to create a new catheter tube for a broader range of use. We designed and produced a three-lumen, 2.5 Fr catheter tube that we introduced to the industry at the 2023 MD&M show in Anaheim. This tube is an authentic 2nd generation of the 1.9 Fr dual lumen. The three-lumen bump tube has the attributes of the two-lumen bump tube, but the scope of its application goes beyond the two lumen and broadens it.
Fenske: What are you doing to remain able to develop and manufacture tubing that meets the ever-increasing demands of medical device manufacturers?
Steele: To stay abreast of the ever-expanding medical technologies that require extruded tubing and profiles, we listen and talk to the people conceiving the ideas that turn into the future medical technologies not yet in existence. Since the beginning of MicroSpec, the company has built a reputation on taking projects others would turn away. Of course, some of these projects did not succeed, but many do and that is how we grow from year to year. We have more than twenty-five high volume tubes/parts that we’ve been extruding for about 25 years or more and they all started out as a concept others had turned away as not worth doing or too complex to consider. We still follow this model of pushing the extrusion envelope and challenging ourselves to innovate to meet the needs of an ever-changing medical device landscape.
Fenske: Why are tubing demands increasing in complexity?

Handling and packaging very small, delicate tubing is technique-sensitive and challenging.
Fenske: In what types of medical applications is “next gen” tubing being used most often?
Steele: “Next gen” tubing is not confined to one or two applications as new development is happening everywhere in the medical device industry. I’ve already given an example of how neonatal tubing is morphing. It’s not just physical design that may define a tube as “next gen”; rather, the materials used for the tube change as well. The polyurethane family of raw materials are often reformulated with various additives to enhance mechanical and physical characteristics to improve performance. (Lubricious and anti-oxidant additives are the most common.)
Fenske: What are the major obstacles that need to be overcome when “pushing the boundaries” for next gen tubing? Is it an issue of equipment, materials, both, or something else entirely?

Very small, complex tubing is challenging to make extrusion tools for.
The challenge of machining the extrusion tools is often the most difficult and generally, the most common hurdle we must clear. Most often, the challenge involves making the machined tools small enough to produce complex tubing that is getting smaller and smaller as medical technology advances. Having a state-of-the-art machine shop is critical, which we have at MicroSpec. Some new tube designs have required us to use wire EDM with wires as small as 0.002 inches, which is a very slow and costly process. On the other hand, it allows us to achieve the intricacies in the tools that enable us to extrude the new tube.

Inspection is often complicated by unusual designs.
For new tubes, we try to define all the challenges in the quoting process, but typically find something not anticipated. Another obstacle we run into is validation of the process and the part. Miniaturized parts are particularly challenging as the specified tolerances are so small the process uses almost the entire allowed tolerance and a 1.33 Cpk cannot be met. In cases like this, an acceptable Cpk needs to be agreed upon with the customer.
Fenske: Do you see areas for further optimization or ways to gain even greater benefits from tubing that’s not yet being utilized?
Steele: Making a new or next generation tube always leads to innovation. Making a new part the first time always shows us what we could have done better and even with parts that meet the customer’s specification the first time, we generally go back to re-work the tools and/or the extrusion process to optimize dimensions. It is our experience a part is not optimized before it has run at least three or four times, but we are always looking to improve the process.
Click here to find out more about MicroSpec >>>>>













