By Sean Fenske, Editor-in-Chief
Robotic surgical systems are gaining significant attention with physicians and hospitals for the many benefits they offer. Helping address surgeon fatigue and offering positioning of multiple instruments at the same time are just two of the advantages presented. But precision accuracy and motion control are attributes the physician shouldn’t even have to consider; they are simply mandatory. As such, development of these systems requires partners who can handle the complexities of such precise movements.
With this in mind, it’s critical for the developers of robotic surgical systems to partner with an experienced motion control specialist to ensure their system meets the needs of their customers. Fortunately, companies like maxon have “cut their teeth” in serving robotic systems for other industries where they were able to evolve their motion control solutions to achieve the exactness required to serve the medical industry.
To share insights on what robotic surgical system developers need to keep in mind and what factors to consider during the design of their technologies, Peter van Beek, Business Development Manager – Medical at maxon precision motors (mpm), responded to a number of questions about the topic. He offers his experience in the following Q&A about these cutting-edge robotic solutions to help ensure future versions continue to move in an exact manner, ensuring successful procedures within the OR.
Sean Fenske: When developing robotic surgical systems, what are some factors engineers should keep in mind about the motion control system?
Peter van Beek: First, determine if the entire robotic system will be built from the ground up, or will market established subassemblies be purchased to make a whole system. Then, define all the surgical procedures that will be possible with your system. Also, early in the design process, establish which parts of the robotic system will need repeated sterilization, as different drive components are selected based on this requirement. Confirm the physical size requirements of the entry point to the body are possible using the existing mechanical systems available on the market. A similar comparative process will need to be done between the torque/speed requirements and currently available drive technology. maxon has supplied drive systems to the surgical robotics industry since its inception and would gladly select, custom design, and contract manufacture drive subassemblies for your specific robotic design.
Fenske: As engineers strive to develop minimally invasive systems, how are you (as the motion provider) helping with this effort?

The EPOS4 Micro 24/5 CAN is a miniaturized OEM positioning controller module for integration into single or multi-axis motion control systems. Designed for use with brushed DC motors with encoders and brushless EC motors (BLDC) with Hall sensors and encoders up to 120 W/360 W.
van Beek: Some of the trending waves in minimally invasive surgery are: overall physically smaller robotic systems, which are less capital intensive; fewer entry points to the body; and surgeries performed by simple and consistently reliable robots. These changes have pushed the “drive assembly” to a smaller physical footprint, while at the same time, still demanding historical torque and speed values. maxon spends eight percent of its annual budget on R&D to continuously increase the power density and efficiencies of their complete drive assemblies. For applications or parts of a robotic system requiring sterilization, there are drive system variants that are autoclavable. In the end, it is maxon’s goal to bring Swiss-designed drive assemblies that are consistently reliable, modular (e.g., motors, gears, brakes sensors, linear actuation, etc.), of the highest power density, in the smallest form factors, and of the lightest weights and highest efficiencies.
Fenske: What advantages are realized by bringing a firm like maxon onto the team to aid with manufacturing of surgical robots?
van Beek: maxon at its core is an engineering company in the business of making world-class drive systems. Our company has been supplying the robotics industry since its beginning, and this long history has provided vast experience that allows the right questions to be asked at the onset of a project. This saves time and optimizes outcomes with the least design cycles. Robotic makers that bring projects to maxon will have the benefit of working with engineers at the sales, project, and R&D levels. Concepts are quickly transitioned in meetings to various design approaches along with timelines to achieve these various ends. As a company, we are focused on new technologies to achieve the best results. Our employees have a vested interest in projects and believe the best outcomes result from partnerships that are born of close collaborative efforts with customers. What we conceptualize together, we build together. In the final review, only concepts we can effectively transition from engineering samples to serial production are considered. Finally, when the design is settled, we can provide the “built to print or specification” contract manufacturing out of our Taunton, Mass., facility.
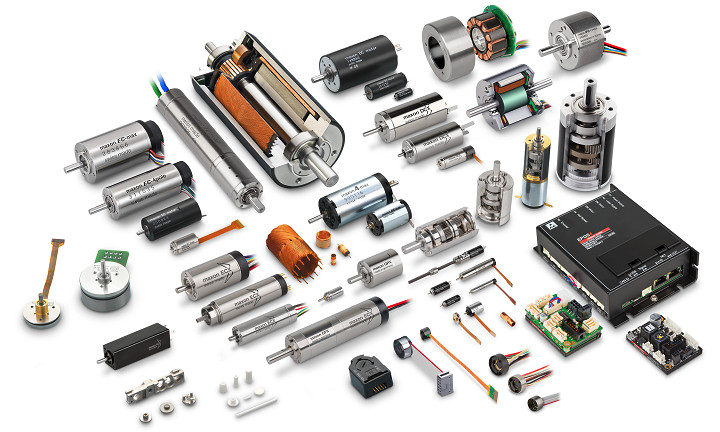
maxon supplies innovative customized drives for any application. A one-stop-shop for precision drive systems: brushed and brushless DC motors, gearheads, and motion controllers.
Fenske: What aspects of the motion system are sometimes overlooked or forgotten in the development process of robotic surgical systems?
van Beek: There are a number of items to keep in mind.
maxon motors are designed with modularity in mind but selecting the correct modules for any particular assembly is critical. Reach out to your local maxon sales engineer for guidance in assembling the various blocks of your best drive assembly for your specific need.
Fenske: How is the experience and expertise of the motion control system provider aiding with unique technical challenges of robotic surgical systems, like a haptic-based interface?

Brushed DCX10 combined with a GPX10 planetary gearhead and ENX encoder. Highly efficient, minimal heat build-up, low noise, and no cogging torque are ideal for use in surgical robots.
van Beek: Our sales engineers can guide you to the correct product lines and motor types within the larger product offerings that provide the best haptic experience based on the physics of the motor construction. maxon’s historic ironless core rotor technology provides linear current-to-torque relationships, allowing a predictable basis upon which to build a true haptic experience. We’ve developed and continue to design the lowest rotor inertia motors possible to achieve the ultimate haptic motor.
Fenske: How does the motion control supplier help with the validation process, such as those required for the EU’s MDR?
van Beek: maxon works closely with its customers to navigate and fulfill all applicable quality standards (ISO9001, ISO13485) and provide documentation as needed to address regulatory needs (FDA or MDR) on a case-by-case basis. maxon has, for many years, been in compliance with EU REACH, WEEE, Waste Framework Directive, and RoHS.
Fenske: Do you have any additional comments you’d like to share based on any of the topics we discussed or something you’d like to tell medical device manufacturers?
van Beek: maxon is now offering engineering services and full contract manufacturing out of its Taunton, Mass., facility. Contact Carsten Horn at Carsten.horn@maxongroup.com for more details.
Learn more about maxon precision motors >>>>>
Robotic surgical systems are gaining significant attention with physicians and hospitals for the many benefits they offer. Helping address surgeon fatigue and offering positioning of multiple instruments at the same time are just two of the advantages presented. But precision accuracy and motion control are attributes the physician shouldn’t even have to consider; they are simply mandatory. As such, development of these systems requires partners who can handle the complexities of such precise movements.
With this in mind, it’s critical for the developers of robotic surgical systems to partner with an experienced motion control specialist to ensure their system meets the needs of their customers. Fortunately, companies like maxon have “cut their teeth” in serving robotic systems for other industries where they were able to evolve their motion control solutions to achieve the exactness required to serve the medical industry.
To share insights on what robotic surgical system developers need to keep in mind and what factors to consider during the design of their technologies, Peter van Beek, Business Development Manager – Medical at maxon precision motors (mpm), responded to a number of questions about the topic. He offers his experience in the following Q&A about these cutting-edge robotic solutions to help ensure future versions continue to move in an exact manner, ensuring successful procedures within the OR.
Sean Fenske: When developing robotic surgical systems, what are some factors engineers should keep in mind about the motion control system?
Peter van Beek: First, determine if the entire robotic system will be built from the ground up, or will market established subassemblies be purchased to make a whole system. Then, define all the surgical procedures that will be possible with your system. Also, early in the design process, establish which parts of the robotic system will need repeated sterilization, as different drive components are selected based on this requirement. Confirm the physical size requirements of the entry point to the body are possible using the existing mechanical systems available on the market. A similar comparative process will need to be done between the torque/speed requirements and currently available drive technology. maxon has supplied drive systems to the surgical robotics industry since its inception and would gladly select, custom design, and contract manufacture drive subassemblies for your specific robotic design.
Fenske: As engineers strive to develop minimally invasive systems, how are you (as the motion provider) helping with this effort?

The EPOS4 Micro 24/5 CAN is a miniaturized OEM positioning controller module for integration into single or multi-axis motion control systems. Designed for use with brushed DC motors with encoders and brushless EC motors (BLDC) with Hall sensors and encoders up to 120 W/360 W.
Fenske: What advantages are realized by bringing a firm like maxon onto the team to aid with manufacturing of surgical robots?
van Beek: maxon at its core is an engineering company in the business of making world-class drive systems. Our company has been supplying the robotics industry since its beginning, and this long history has provided vast experience that allows the right questions to be asked at the onset of a project. This saves time and optimizes outcomes with the least design cycles. Robotic makers that bring projects to maxon will have the benefit of working with engineers at the sales, project, and R&D levels. Concepts are quickly transitioned in meetings to various design approaches along with timelines to achieve these various ends. As a company, we are focused on new technologies to achieve the best results. Our employees have a vested interest in projects and believe the best outcomes result from partnerships that are born of close collaborative efforts with customers. What we conceptualize together, we build together. In the final review, only concepts we can effectively transition from engineering samples to serial production are considered. Finally, when the design is settled, we can provide the “built to print or specification” contract manufacturing out of our Taunton, Mass., facility.

maxon supplies innovative customized drives for any application. A one-stop-shop for precision drive systems: brushed and brushless DC motors, gearheads, and motion controllers.
Fenske: What aspects of the motion system are sometimes overlooked or forgotten in the development process of robotic surgical systems?
van Beek: There are a number of items to keep in mind.
- Be sure to analyze and address heating issues that can result from a drive system being placed in closed environments.
- Determine which drive assemblies will need sterilization early in the design process.
- To avoid cross talk between power and sensor cabling, attempt to keep motor and sensor cables separated.
- Avoid modification costs associated with unnecessary customization.
- Determine each axis’ requirement for back drivability or to hold position should power be lost.
- Utilizing catalog nominal power values in the selection of a drive can result in an undersized motor. Instead, select motors based on required torque capacity.
- Determine the need for sealing or autoclave for all drive assemblies.
- Fully understanding the duty cycle of a particular axis may allow a smaller drive to be utilized on that particular axis.
- Consider redundant sensor design (on motor and output) and associated controls to provide the precision positional accuracy that avoids undesired overshooting or oscillations.
- Analyze the complete drive assembly mechanism to confirm it complies with creepage and clearance applicable standards, and that current paths are fully understood as it relates to electric shock.
maxon motors are designed with modularity in mind but selecting the correct modules for any particular assembly is critical. Reach out to your local maxon sales engineer for guidance in assembling the various blocks of your best drive assembly for your specific need.
Fenske: How is the experience and expertise of the motion control system provider aiding with unique technical challenges of robotic surgical systems, like a haptic-based interface?

Brushed DCX10 combined with a GPX10 planetary gearhead and ENX encoder. Highly efficient, minimal heat build-up, low noise, and no cogging torque are ideal for use in surgical robots.
Fenske: How does the motion control supplier help with the validation process, such as those required for the EU’s MDR?
van Beek: maxon works closely with its customers to navigate and fulfill all applicable quality standards (ISO9001, ISO13485) and provide documentation as needed to address regulatory needs (FDA or MDR) on a case-by-case basis. maxon has, for many years, been in compliance with EU REACH, WEEE, Waste Framework Directive, and RoHS.
Fenske: Do you have any additional comments you’d like to share based on any of the topics we discussed or something you’d like to tell medical device manufacturers?
van Beek: maxon is now offering engineering services and full contract manufacturing out of its Taunton, Mass., facility. Contact Carsten Horn at Carsten.horn@maxongroup.com for more details.
Learn more about maxon precision motors >>>>>














