Dylan Howes, Applications Engineer, TT Electronics01.29.20
With the growth of an aging population and the rising cost of hospital-based care, advanced technologies are being developed to facilitate remote healthcare. Moving healthcare out of the hospital and into the home offers patients, healthcare providers, and insurers both convenience and cost savings. A challenge arises, however, for regulatory authorities (and OEMs) in determining appropriate safety considerations for these technologies. Unlike hospitals, patients’ homes are uncontrolled environments. There are no trained equipment operators or guarantee of a proper electrical installation.
Accordingly, additional measures must be taken to ensure equipment remains safe for use in these environments. To standardize these additional measures, a group of clinicians, engineers, and regulators established the 60601-1-11 collateral requirements for medical electrical equipment and systems used in the home healthcare environment.
Provisions of the standard impose several constraints on the selection of AC/DC power converters for home healthcare applications. As OEMs endeavor to bring equipment originally designed for use in a clinical environment to the home, they often learn a single converter will not suffice for both environments. It’s important to understand how requirements of the collateral standard impact the selection of an AC/DC power converter for the home and what constraints to consider when using a 60601-1 general standard certified converter.
Low-Line Operation to 85 Percent of Nominal
The 60601-1 general standard assumes supply mains are expected to have a 10 percent tolerance from nominal. That is, if a device is to be rated for operation on supply mains ranging from 100 to 240 VAC (Universal Line), the device must safely function on input voltages ranging from 90 to 264 VAC. Mains voltages in the home may be less predictable. For this reason, the 60601-1-11 collateral standard assumes nominal mains voltages may exhibit a tolerance of +10 percent/-15 percent, corresponding to an operational input voltage range of 85 to 264 VAC for universal line input devices.
The first impact the lower-line operation requirement has on the power converter is the presence of greater primary-side currents. A converter’s input current can be modeled as a function of output power, efficiency, power factor, and line-voltage:
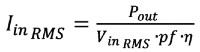
Where Pout is the output power in Watts, pf is the power factor, and η is the conversion efficiency. If one assumes efficiency and power factor are both independent of line voltage (which is generally not true but simplifies this discussion without muddying the implications) then, for a given load, the input current is inversely proportional to the line voltage.
Accordingly, a 5.55 percent reduction in line voltage (90 VAC down to 85 VAC) results in roughly a 5.9 percent increase in line current. The key observation is that any current margin or thermal (I2R) margin designed into the primary side components has been significantly reduced if the converter was designed for a minimum line voltage of 90 VAC.
Increased primary side currents are not the only concern. Typically, the input voltage is directly related to the amount of energy per cycle the converter is physically able to transfer to secondary. A reduction in input voltage (while made up by current hikes from a power perspective) can ultimately reduce the amount of energy that can be transferred per cycle. When the input voltage is reduced below the anticipated minimum, there is a risk of maxing out the switching frequency and/or duty cycle. In such a case, the converter will be rendered unable to sustain its rated load.
The solution for a 60601-1 general standard certified power supply unit (PSU; designed for 90-264 VAC operation) is output power derating. It is trivial to calculate the output power reduction required to offset the increases in primary side currents according to the aforementioned formula, but a bit more difficult to anticipate effects on the actual conversion process. Inquire with the PSU manufacturer regarding an appropriate low-line derating strategy.
Class II Construction
Power converters must provide users with at least two means of protection from hazardous voltages. Class I power supplies protect users via a single layer of basic insulation and a protective Earth ground connection, which keeps users safe by providing a low impedance path to zero potential for fault currents to follow. The PSU manufacturer’s ability to ensure that path has an appropriately low impedance ends at the PSU AC inlet. Earth ground connections in older homes are notoriously unreliable (if existent) and can be easily bypassed by users. IEC 60601-1-11 does not allow OEMs to rely on in-home Earth ground connections for one of the two required protection mechanisms. Rather, the collateral standard requires power supplies be of construction Class II.
Class II power supplies utilize additional basic insulation or reinforced insulation to ensure that with or without a reliable Earth ground connection, users are properly protected from hazardous voltages.
BF Class Output
Clause 6 of the collateral standard mandates if the medical electrical equipment is equipped with applied parts, those parts must be type BF at minimum. While a BF ready power supply is not necessarily required to facilitate the use of type BF applied parts, it can make the process simpler. It is important to recognize power supplies themselves are not applied parts and, therefore, do not have an applied part type directly associated with them. However, the PSU can be selected to provide the required degree of isolation from ground and to properly mitigate leakage currents. Patient auxiliary currents must be constrained to 100 µAac (500 µAac under single fault conditions) and not all 60601-1 certified power supplies offer BF class outputs.
As was detailed in the previous section, power supplies for home healthcare applications must be of construction Class II, so the Earth ground isolation component of this characterization is moot. Patient auxiliary currents, on the other hand, must be constrained to 100 µAac (500 µAac under single fault conditions). Not all 60601-1 certified power supplies offer BF class outputs.
IP21 Rated Ingress Protection
Per 60601-1-11, external power adaptors must be encased in a manner that provides at least IP21 rated ingress protection in accordance with IEC 60529, and must be labeled accordingly. One would be hard pressed to find a power adaptor that was not inherently solid ingress Level 2 (IP2x) compliant. The same is not true for IPx1, Level 1 liquid ingress protection.
While the IEC 60529 test for IPx1, stipulating a drip rate of 1 mm/min (like a light rainfall) is seemingly benign, over the 10-minute test duration one may be surprised to find water dripping off the edges of a power supply enclosure can pool up at interfaces with cable strain reliefs, or along the edges of a removable AC blade attachment, and make its way into the electronics. This would constitute a failure and an inadequacy for use in a home healthcare application. Achieving IPx1 is not a difficult engineering problem to solve, and many off-the-shelf 60601-1 power supplies are inherently compliant, but one should be careful not to assume compliance.
Augmented Mechanical Strength
Medical electrical equipment used in an uncontrolled home environment is more likely to be subjected to mechanical disturbances than equipment used in a clinical environment. Playing children or rogue pets may inadvertently knock a device from a tabletop. A weekend away from home may require transport of home healthcare equipment in the trunk of a car traveling over speed bumps and rumble strips. There is a clear need for the equipment to remain safe and functional in the face of augmented mechanical stress.
Basic safety and essential performance of a home healthcare power supply must be maintained after undergoing the following shock and broadband random vibration tests:
Shock: Tested against IEC 60068-2-27:2008 with three half-sine pulses of 150 m/s2 peak acceleration and 11 ms duration through each axis.
Vibration: Tested against IEC 60068-2-64:2008 for 30 minutes per each perpendicular axis with the following acceleration amplitude:
10 Hz to 100 Hz: 1.0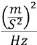
200 Hz to 200 Hz: -3db per octave
200 Hz to 2,000 Hz: 0.5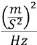
This is another example of a 60601-1-11 item that may very well be inherent to several well-designed general standard certified PSUs, but inherent compliance should not be assumed.
60601-1-2 Edition 4—Electromagnetic Compatibility
Power supplies for use in home healthcare applications must be compliant with IEC 60601-1-2 Edition 4. In addition to some nuanced deviations in emissions mandates over Edition 3, Edition 4 imposes some stringent immunity requirements including ESD contact discharge immunity of ±8 kV; ESD air discharge immunity of ±15 kV; radiated immunity for fields of strength up to 10 V/m and across a broader frequency range (80 Mhz to 2.7 GHz); mandated 100 kHz repetition rate for electric fast transients; test levels unchanged; tenfold increase in power frequency magnetic field immunity to 30 A/m; single cycle, 100 percent reduction voltage interruption test.
Your Next Design
When bringing your device to the home healthcare market, IEC 60601-1-11 imposes an additional 13 clauses over the 60601-1 general standard. A number of these design challenges are directly associated with careful selection of an appropriate power converter. By selecting a partner that is well versed in the nuances of the IEC 60601-1-11 standard as they pertain to power system requirements, your team can tap into expertise that will guide you well in your home healthcare power supply selection.
Dylan Howes, applications engineer at TT Electronics, manages all technical aspects of power converter design-in efforts, and authors technical content featuring power conversion trends and technologies. Connect with him at Dylan.Howes@ttelectronics.com.
Accordingly, additional measures must be taken to ensure equipment remains safe for use in these environments. To standardize these additional measures, a group of clinicians, engineers, and regulators established the 60601-1-11 collateral requirements for medical electrical equipment and systems used in the home healthcare environment.
Provisions of the standard impose several constraints on the selection of AC/DC power converters for home healthcare applications. As OEMs endeavor to bring equipment originally designed for use in a clinical environment to the home, they often learn a single converter will not suffice for both environments. It’s important to understand how requirements of the collateral standard impact the selection of an AC/DC power converter for the home and what constraints to consider when using a 60601-1 general standard certified converter.
Low-Line Operation to 85 Percent of Nominal
The 60601-1 general standard assumes supply mains are expected to have a 10 percent tolerance from nominal. That is, if a device is to be rated for operation on supply mains ranging from 100 to 240 VAC (Universal Line), the device must safely function on input voltages ranging from 90 to 264 VAC. Mains voltages in the home may be less predictable. For this reason, the 60601-1-11 collateral standard assumes nominal mains voltages may exhibit a tolerance of +10 percent/-15 percent, corresponding to an operational input voltage range of 85 to 264 VAC for universal line input devices.
The first impact the lower-line operation requirement has on the power converter is the presence of greater primary-side currents. A converter’s input current can be modeled as a function of output power, efficiency, power factor, and line-voltage:
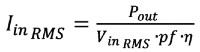
Where Pout is the output power in Watts, pf is the power factor, and η is the conversion efficiency. If one assumes efficiency and power factor are both independent of line voltage (which is generally not true but simplifies this discussion without muddying the implications) then, for a given load, the input current is inversely proportional to the line voltage.
Accordingly, a 5.55 percent reduction in line voltage (90 VAC down to 85 VAC) results in roughly a 5.9 percent increase in line current. The key observation is that any current margin or thermal (I2R) margin designed into the primary side components has been significantly reduced if the converter was designed for a minimum line voltage of 90 VAC.
Increased primary side currents are not the only concern. Typically, the input voltage is directly related to the amount of energy per cycle the converter is physically able to transfer to secondary. A reduction in input voltage (while made up by current hikes from a power perspective) can ultimately reduce the amount of energy that can be transferred per cycle. When the input voltage is reduced below the anticipated minimum, there is a risk of maxing out the switching frequency and/or duty cycle. In such a case, the converter will be rendered unable to sustain its rated load.
The solution for a 60601-1 general standard certified power supply unit (PSU; designed for 90-264 VAC operation) is output power derating. It is trivial to calculate the output power reduction required to offset the increases in primary side currents according to the aforementioned formula, but a bit more difficult to anticipate effects on the actual conversion process. Inquire with the PSU manufacturer regarding an appropriate low-line derating strategy.
Class II Construction
Power converters must provide users with at least two means of protection from hazardous voltages. Class I power supplies protect users via a single layer of basic insulation and a protective Earth ground connection, which keeps users safe by providing a low impedance path to zero potential for fault currents to follow. The PSU manufacturer’s ability to ensure that path has an appropriately low impedance ends at the PSU AC inlet. Earth ground connections in older homes are notoriously unreliable (if existent) and can be easily bypassed by users. IEC 60601-1-11 does not allow OEMs to rely on in-home Earth ground connections for one of the two required protection mechanisms. Rather, the collateral standard requires power supplies be of construction Class II.
Class II power supplies utilize additional basic insulation or reinforced insulation to ensure that with or without a reliable Earth ground connection, users are properly protected from hazardous voltages.
BF Class Output
Clause 6 of the collateral standard mandates if the medical electrical equipment is equipped with applied parts, those parts must be type BF at minimum. While a BF ready power supply is not necessarily required to facilitate the use of type BF applied parts, it can make the process simpler. It is important to recognize power supplies themselves are not applied parts and, therefore, do not have an applied part type directly associated with them. However, the PSU can be selected to provide the required degree of isolation from ground and to properly mitigate leakage currents. Patient auxiliary currents must be constrained to 100 µAac (500 µAac under single fault conditions) and not all 60601-1 certified power supplies offer BF class outputs.
As was detailed in the previous section, power supplies for home healthcare applications must be of construction Class II, so the Earth ground isolation component of this characterization is moot. Patient auxiliary currents, on the other hand, must be constrained to 100 µAac (500 µAac under single fault conditions). Not all 60601-1 certified power supplies offer BF class outputs.
IP21 Rated Ingress Protection
Per 60601-1-11, external power adaptors must be encased in a manner that provides at least IP21 rated ingress protection in accordance with IEC 60529, and must be labeled accordingly. One would be hard pressed to find a power adaptor that was not inherently solid ingress Level 2 (IP2x) compliant. The same is not true for IPx1, Level 1 liquid ingress protection.
While the IEC 60529 test for IPx1, stipulating a drip rate of 1 mm/min (like a light rainfall) is seemingly benign, over the 10-minute test duration one may be surprised to find water dripping off the edges of a power supply enclosure can pool up at interfaces with cable strain reliefs, or along the edges of a removable AC blade attachment, and make its way into the electronics. This would constitute a failure and an inadequacy for use in a home healthcare application. Achieving IPx1 is not a difficult engineering problem to solve, and many off-the-shelf 60601-1 power supplies are inherently compliant, but one should be careful not to assume compliance.
Augmented Mechanical Strength
Medical electrical equipment used in an uncontrolled home environment is more likely to be subjected to mechanical disturbances than equipment used in a clinical environment. Playing children or rogue pets may inadvertently knock a device from a tabletop. A weekend away from home may require transport of home healthcare equipment in the trunk of a car traveling over speed bumps and rumble strips. There is a clear need for the equipment to remain safe and functional in the face of augmented mechanical stress.
Basic safety and essential performance of a home healthcare power supply must be maintained after undergoing the following shock and broadband random vibration tests:
Shock: Tested against IEC 60068-2-27:2008 with three half-sine pulses of 150 m/s2 peak acceleration and 11 ms duration through each axis.
Vibration: Tested against IEC 60068-2-64:2008 for 30 minutes per each perpendicular axis with the following acceleration amplitude:
10 Hz to 100 Hz: 1.0
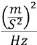
200 Hz to 200 Hz: -3db per octave
200 Hz to 2,000 Hz: 0.5
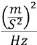
This is another example of a 60601-1-11 item that may very well be inherent to several well-designed general standard certified PSUs, but inherent compliance should not be assumed.
60601-1-2 Edition 4—Electromagnetic Compatibility
Power supplies for use in home healthcare applications must be compliant with IEC 60601-1-2 Edition 4. In addition to some nuanced deviations in emissions mandates over Edition 3, Edition 4 imposes some stringent immunity requirements including ESD contact discharge immunity of ±8 kV; ESD air discharge immunity of ±15 kV; radiated immunity for fields of strength up to 10 V/m and across a broader frequency range (80 Mhz to 2.7 GHz); mandated 100 kHz repetition rate for electric fast transients; test levels unchanged; tenfold increase in power frequency magnetic field immunity to 30 A/m; single cycle, 100 percent reduction voltage interruption test.
Your Next Design
When bringing your device to the home healthcare market, IEC 60601-1-11 imposes an additional 13 clauses over the 60601-1 general standard. A number of these design challenges are directly associated with careful selection of an appropriate power converter. By selecting a partner that is well versed in the nuances of the IEC 60601-1-11 standard as they pertain to power system requirements, your team can tap into expertise that will guide you well in your home healthcare power supply selection.
Dylan Howes, applications engineer at TT Electronics, manages all technical aspects of power converter design-in efforts, and authors technical content featuring power conversion trends and technologies. Connect with him at Dylan.Howes@ttelectronics.com.