Business Wire10.27.20
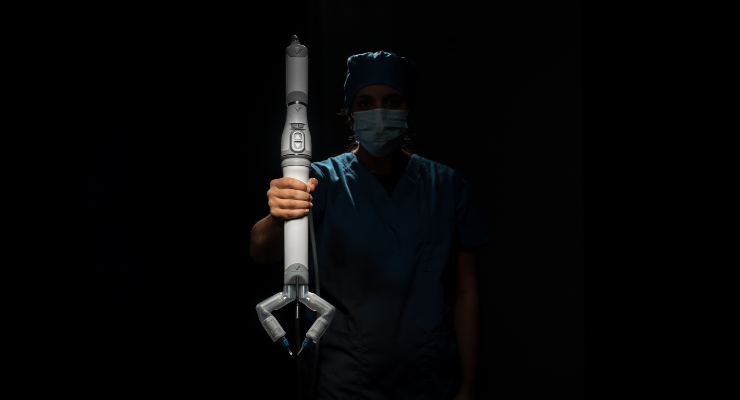
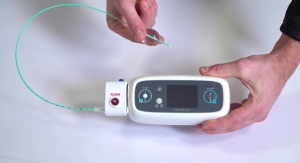
Virtual Incision Corporation, a medical device company pioneering a first-of-its-kind miniaturized surgical platform, has received an Investigational Device Exemption (IDE) from the U.S. Food and Drug Administration (FDA) for the company’s MIRA (“miniaturized in vivo robotic assistant”) Platform.
The IDE will allow the company to initiate a clinical study of MIRA at a limited number of U.S. hospitals in support of the system’s regulatory pathway to approval.
“Because of its clear benefits for patients, the demand for robotically-assisted surgery continues to increase, though challenges still inhibit broader adoption. MIRA is intended to overcome these limitations, with a simple and cost-effective solution that offers the potential to bring minimally invasive laparoscopic surgery to many more patients,” said John Murphy, president and CEO of Virtual Incision. “The IDE approval for MIRA is an exciting and critical next step that will allow us to evaluate the safety and effectiveness of this novel device in patients. We look forward to working closely with the surgical teams and study sites to advance the MIRA surgical platform with the goal of making minimally invasive surgery more accessible.”
Colorectal and lower gastrointestinal procedures are among the fastest growing surgeries in the U.S., with more than 400,000 colon resection procedures performed each year. Minimally invasive colectomies have been shown to reduce mortality, and can also reduce a patient’s healing time, pain, complications, and hospital readmissions. Manual laparoscopic colectomies, though also minimally invasive, can be difficult to perform and can have less than ideal cosmetic outcomes.
“Many benign and malignant conditions require removal of a portion of the colon. Currently the most common approach is open surgery, which involves a very large incision, a long hospital stay and several weeks of recovery,” said Dr. Dmitry Oleynikov, co-founder and chief medical officer of Virtual Incision. “Open colectomies carry a high risk of surgical site infection and other complications that can negatively affect a patient’s quality of life.”
“Despite significant technological advancements and improved patient outcomes that can reduce the total cost of care, adoption of minimally invasive colon surgery has been limited,” said Shane Farritor, Ph.D., Virtual Incision’s co-founder and chief technology officer. “This milestone is a step toward a miniaturized solution to robotically-assisted laparoscopic surgery.”
The IDE will allow the company to initiate a clinical study of MIRA at a limited number of U.S. hospitals in support of the system’s regulatory pathway to approval.
“Because of its clear benefits for patients, the demand for robotically-assisted surgery continues to increase, though challenges still inhibit broader adoption. MIRA is intended to overcome these limitations, with a simple and cost-effective solution that offers the potential to bring minimally invasive laparoscopic surgery to many more patients,” said John Murphy, president and CEO of Virtual Incision. “The IDE approval for MIRA is an exciting and critical next step that will allow us to evaluate the safety and effectiveness of this novel device in patients. We look forward to working closely with the surgical teams and study sites to advance the MIRA surgical platform with the goal of making minimally invasive surgery more accessible.”
Colorectal and lower gastrointestinal procedures are among the fastest growing surgeries in the U.S., with more than 400,000 colon resection procedures performed each year. Minimally invasive colectomies have been shown to reduce mortality, and can also reduce a patient’s healing time, pain, complications, and hospital readmissions. Manual laparoscopic colectomies, though also minimally invasive, can be difficult to perform and can have less than ideal cosmetic outcomes.
“Many benign and malignant conditions require removal of a portion of the colon. Currently the most common approach is open surgery, which involves a very large incision, a long hospital stay and several weeks of recovery,” said Dr. Dmitry Oleynikov, co-founder and chief medical officer of Virtual Incision. “Open colectomies carry a high risk of surgical site infection and other complications that can negatively affect a patient’s quality of life.”
“Despite significant technological advancements and improved patient outcomes that can reduce the total cost of care, adoption of minimally invasive colon surgery has been limited,” said Shane Farritor, Ph.D., Virtual Incision’s co-founder and chief technology officer. “This milestone is a step toward a miniaturized solution to robotically-assisted laparoscopic surgery.”