Cepheid Developing Combination Test for SARS-CoV-2, Flu A/B, and RSV
By PR Newswire | 06.09.20
Four viral infections have similar patient presentations; accurate detection and differentiation critical next flu season.
Cepheid announced the development of a next-generation test to assist global efforts in the fight against the spread of COVID-19 during the upcoming respiratory virus season. The Xpert Xpress SARS-CoV-2/Flu/RSV four-in-one test is expected to deliver qualitative detection of SARS-CoV-2, Flu A, Flu B and RSV from a single patient sample. The test is designed for use on any of Cepheid's more than 25,000 GeneXpert Systems placed worldwide, with results expected in as little as 35 minutes.
"Patients infected by SARS-CoV-2, Flu A, Flu B, and RSV have overlapping clinical presentations, but fundamentally different treatment and management pathways," said Dr. David Persing, MD, Ph.D., Chief Medical and Technology Officer at Cepheid. "Unlike the common cold viruses, infection with these four viruses is often associated with fever and other systemic manifestations that may be coupled with severe outcomes, especially in the elderly."
Curious how medtech's top companies are responding to the COVID-19 pandemic? Get insights into each company's actions by clicking here.
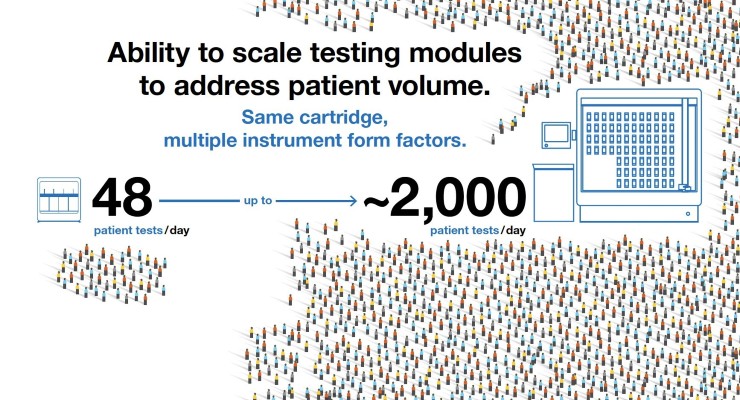
In the coming weeks, Cepheid intends to pursue the FDA's Emergency Use Authorization (EUA) pathway for regulatory authorization and make the test available globally on its cartridge-based GeneXpert Systems, which features instruments that can be configured for both near patient point-of-care and high volume laboratory testing needs.
"On March 20th, Cepheid was granted the first-ever EUA by the FDA for use in point-of-care settings for our Xpert Xpress SARS-CoV-2 test," said Cepheid President Warren Kocmond. "Since then, we have experienced unprecedented demand for this technology. Leveraging the quality design of Xpert Xpress SARS-CoV-2 and our widely utilized Xpert Xpress Flu/RSV tests, we're combining two world-class products in a single, rapid solution ahead of the upcoming flu season. This will enable our customers to have increased testing throughput on their current GeneXpert System and increase our ability to provide supply continuity for the market."
"Patients infected by SARS-CoV-2, Flu A, Flu B, and RSV have overlapping clinical presentations, but fundamentally different treatment and management pathways," said Dr. David Persing, MD, Ph.D., Chief Medical and Technology Officer at Cepheid. "Unlike the common cold viruses, infection with these four viruses is often associated with fever and other systemic manifestations that may be coupled with severe outcomes, especially in the elderly."
Curious how medtech's top companies are responding to the COVID-19 pandemic? Get insights into each company's actions by clicking here.
In the coming weeks, Cepheid intends to pursue the FDA's Emergency Use Authorization (EUA) pathway for regulatory authorization and make the test available globally on its cartridge-based GeneXpert Systems, which features instruments that can be configured for both near patient point-of-care and high volume laboratory testing needs.
"On March 20th, Cepheid was granted the first-ever EUA by the FDA for use in point-of-care settings for our Xpert Xpress SARS-CoV-2 test," said Cepheid President Warren Kocmond. "Since then, we have experienced unprecedented demand for this technology. Leveraging the quality design of Xpert Xpress SARS-CoV-2 and our widely utilized Xpert Xpress Flu/RSV tests, we're combining two world-class products in a single, rapid solution ahead of the upcoming flu season. This will enable our customers to have increased testing throughput on their current GeneXpert System and increase our ability to provide supply continuity for the market."