BD, BioMedomics Launch Test to Detect Exposure to COVID-19
By BD | 03.31.20
Point-of-care blood test detects evidence of present or past exposure in 15 minutes.
BD (Becton, Dickinson and Company), a leading global medical technology company, and BioMedomics, a North Carolina-based clinical diagnostics company, have announced the release of a new point-of-care test that can detect antibodies in blood to confirm current or past exposure to COVID-19 in as little as 15 minutes.
The new test, developed and manufactured by BioMedomics, will be available through BD and distributed exclusively by Henry Schein Inc. to health care providers throughout the United States.
How It Works
The test does not require special equipment and may be used in a laboratory or at the point of care. The test detects antibodies in the blood that are produced by the body in response to coronavirus infection. These antibodies are typically present in the middle to later stages of COVID-19 infection, but may remain present after exposure, which helps clinicians determine who has been exposed to the coronavirus, even if a person didn't exhibit any symptoms of the COVID-19 disease. Data on past exposure is important for researchers to more accurately understand the likely true occurrence of SARS-CoV-2 infection across a population. This information will be helpful in informing future strategies for combatting COVID-19.
"Serology tests are important because they provide an additional piece of information to aid in characterizing possible prior exposure to SARS-CoV-2, especially since many infections are mild or asymptomatic in severity," said Dave Hickey, president of Integrated Diagnostic Solutions for BD. "Initial evidence suggests that nearly all patients infected with SARS-CoV-2 will have developed a detectable antibody response within days of symptom onset, at which time a negative serologic test, along with molecular diagnostics, could be helpful in ruling out COVID-19."
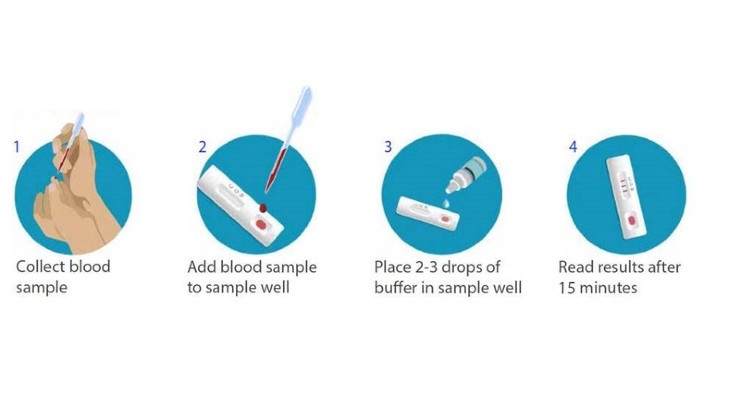
The test is completed in four, simple steps. First, blood is collected through normal blood collection devices such as the BD Microtainer Contact-Activated Lancet. A few drops of blood are then transferred to the test cartridge, followed by two to three drops of a buffer. The results can be read in 15 minutes, similar to how over-the-counter pregnancy tests show multiple lines for positive results and a single line for negative results.
"BioMedomics designed the test to be easy to use and provide results in minutes, with no special equipment necessary or the need to transport the sample to a laboratory for analysis," said Frank Wang, CEO of BioMedomics. "Our test has been clinically validated at several hospitals and clinical laboratories in both the U.S. and China, and our published clinical data in the Journal of Medical Virology was one of the world's first for a COVID-19 serology test. It has been used widely in China during the COVID-19 outbreak and is now ready to help combat coronavirus in the U.S. through our collaboration with BD. We are committed to doing our part to battle this disease and are excited to have BD as a partner to help deliver our high-quality rapid test to those who need it most."
The test has not been reviewed by the FDA but is permitted for distribution and use under the public health emergency guidance issued by FDA on March 16, 2020, and BD expects to begin shipping tests in April. BD will have capacity to supply more than one million tests over the coming months, with the ability to scale up based on market demand and is working with medical products distribution company Henry Schein to make these tests available to medical care facilities throughout the United States. Health care providers can order the test and all collection devices needed to perform the test by contacting their BD or Henry Schein representatives.
The new test, developed and manufactured by BioMedomics, will be available through BD and distributed exclusively by Henry Schein Inc. to health care providers throughout the United States.
How It Works
The test does not require special equipment and may be used in a laboratory or at the point of care. The test detects antibodies in the blood that are produced by the body in response to coronavirus infection. These antibodies are typically present in the middle to later stages of COVID-19 infection, but may remain present after exposure, which helps clinicians determine who has been exposed to the coronavirus, even if a person didn't exhibit any symptoms of the COVID-19 disease. Data on past exposure is important for researchers to more accurately understand the likely true occurrence of SARS-CoV-2 infection across a population. This information will be helpful in informing future strategies for combatting COVID-19.
"Serology tests are important because they provide an additional piece of information to aid in characterizing possible prior exposure to SARS-CoV-2, especially since many infections are mild or asymptomatic in severity," said Dave Hickey, president of Integrated Diagnostic Solutions for BD. "Initial evidence suggests that nearly all patients infected with SARS-CoV-2 will have developed a detectable antibody response within days of symptom onset, at which time a negative serologic test, along with molecular diagnostics, could be helpful in ruling out COVID-19."
The test is completed in four, simple steps. First, blood is collected through normal blood collection devices such as the BD Microtainer Contact-Activated Lancet. A few drops of blood are then transferred to the test cartridge, followed by two to three drops of a buffer. The results can be read in 15 minutes, similar to how over-the-counter pregnancy tests show multiple lines for positive results and a single line for negative results.
"BioMedomics designed the test to be easy to use and provide results in minutes, with no special equipment necessary or the need to transport the sample to a laboratory for analysis," said Frank Wang, CEO of BioMedomics. "Our test has been clinically validated at several hospitals and clinical laboratories in both the U.S. and China, and our published clinical data in the Journal of Medical Virology was one of the world's first for a COVID-19 serology test. It has been used widely in China during the COVID-19 outbreak and is now ready to help combat coronavirus in the U.S. through our collaboration with BD. We are committed to doing our part to battle this disease and are excited to have BD as a partner to help deliver our high-quality rapid test to those who need it most."
The test has not been reviewed by the FDA but is permitted for distribution and use under the public health emergency guidance issued by FDA on March 16, 2020, and BD expects to begin shipping tests in April. BD will have capacity to supply more than one million tests over the coming months, with the ability to scale up based on market demand and is working with medical products distribution company Henry Schein to make these tests available to medical care facilities throughout the United States. Health care providers can order the test and all collection devices needed to perform the test by contacting their BD or Henry Schein representatives.